Steroids in chronic subdural hematomas (SUCRE trial): study protocol for a randomized controlled trial
- PMID: 28583162
- PMCID: PMC5460366
- DOI: 10.1186/s13063-017-1990-8
Steroids in chronic subdural hematomas (SUCRE trial): study protocol for a randomized controlled trial
Abstract
Background: Chronic subdural hematoma (CSDH) is a common neurological pathology, especially in older patients. The actual "gold standard" of treatment is surgical evacuation, with various techniques used across neurosurgical teams. Over the years, there has been growing evidence that inflammatory processes play a major role in the pathogenesis of CSDH. In that context, the use of corticosteroids has been proposed alone or as an adjuvant treatment to surgery. However, this practice remains very empirical and there is a need for high-quality-of-evidence studies to clarify the role of corticosteroids in the management of CSDH.
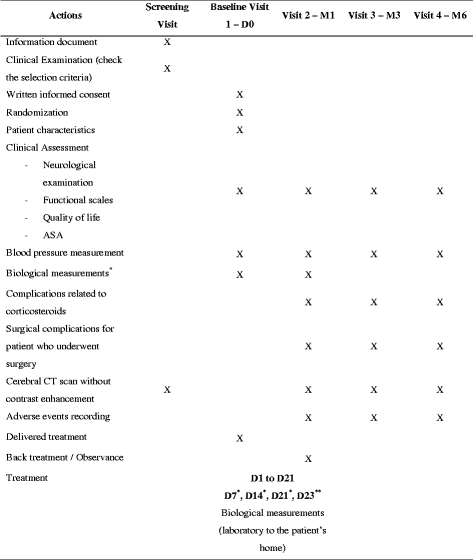
Methods/design: We propose a double-blind, randomized controlled trial comparing methylprednisolone versus placebo in the treatment of CSDH without clinical and/or radiological signs of severity. The treatment will be administered daily for a duration of 3 weeks, at a dose of 1 mg/kg. The primary endpoint will be the delay of occurrence of surgical treatment at 1 month following the introduction of the treatment. Secondary endpoints will include the rate of recourse to surgery, survival rate, quality of life and functional assessments, occurrence of systemic secondary effects and radiological assessment of the response to treatment. This multimodal assessment will be done at 1, 3 and 6 months. Two hundred and two patients (101 per arm) are expected to be included considering our primary hypotheses.
Discussion: This trial started in June 2016; its results may open interesting alternatives to surgery in the management of patients harboring a CSDH, and may provide insights into the natural history of this common pathology.
Trial registration: ClinicalTrials.gov, ID: NCT02650609 . Registered on 4 January 2016. Graphical output of the OBF boundaries.
Keywords: Chronic subdural hematoma; Conservative treatment; Corticosteroids; Elderly patients; Methylprednisolone.
Figures
References
-
- Santarius T, Kirkpatrick PJ, Kolias AG, Hutchinson PJ. Working toward rational and evidence-based treatment of chronic subdural hematoma. Clin Neurosurg. 2010;57:112–22. - PubMed
-
- Bourgeois P, Sleiman M, Louis E, Haddad E, Touzet G, Fichten A, Lejeune JP. Chronic subdural hematoma in patients over 80 years of age. Neurochirurgie. 1999;45:124–8. - PubMed
-
- Cousseau DH, Echevarría Martín G, Gaspari M, Gonorazky SE. Chronic and subacute subdural haematoma. An epidemiological study in a captive population. Rev Neurol. 2001;32:821–4. - PubMed
-
- Kinsella K, Velkoff V. An aging world: 2001. Washington DC: US Government Printing Office; 2001.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical