Follistatin is a metastasis suppressor in a mouse model of HER2-positive breast cancer
- PMID: 28583174
- PMCID: PMC5460489
- DOI: 10.1186/s13058-017-0857-y
Follistatin is a metastasis suppressor in a mouse model of HER2-positive breast cancer
Abstract
Background: Follistatin (FST) is an intrinsic inhibitor of activin, a member of the transforming growth factor-β superfamily of ligands. The prognostic value of FST and its family members, the follistatin-like (FSTL) proteins, have been studied in various cancers. However, these studies, as well as limited functional analyses of the FSTL proteins, have yielded conflicting results on the role of these proteins in disease progression. Furthermore, very few have been focused on FST itself. We assessed whether FST may be a suppressor of tumorigenesis and/or metastatic progression in breast cancer.
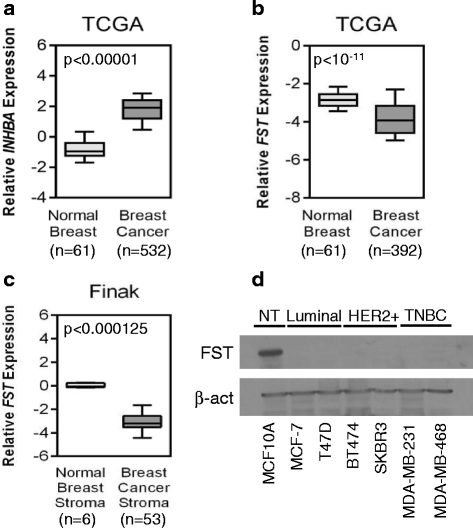
Methods: Using publicly available gene expression data, we examined the expression patterns of FST and INHBA, a subunit of activin, in normal and cancerous breast tissue and the prognostic value of FST in breast cancer metastases, recurrence-free survival, and overall survival. The functional effects of activin and FST on in vitro proliferation, migration, and invasion of breast cancer cells were also examined. FST overexpression in an autochthonous mouse model of breast cancer was then used to assess the in vivo impact of FST on metastatic progression.
Results: Examination of multiple breast cancer datasets revealed that FST expression is reduced in breast cancers compared with normal tissue and that low FST expression predicts increased metastasis and reduced overall survival. FST expression was also reduced in a mouse model of HER2/Neu-induced metastatic breast cancer. We found that FST blocks activin-induced breast epithelial cell migration in vitro, suggesting that its loss may promote breast cancer aggressiveness. To directly determine if FST restoration could inhibit metastatic progression, we transgenically expressed FST in the HER2/Neu model. Although FST had no impact on tumor initiation or growth, it completely blocked the formation of lung metastases.
Conclusions: These data indicate that FST is a bona fide metastasis suppressor in this mouse model and support future efforts to develop an FST mimetic to suppress metastatic progression.
Keywords: Activin; Breast cancer; Follistatin; Metastasis; Migration.
Figures



Similar articles
-
Two distinct mTORC2-dependent pathways converge on Rac1 to drive breast cancer metastasis.Breast Cancer Res. 2017 Jun 30;19(1):74. doi: 10.1186/s13058-017-0868-8. Breast Cancer Res. 2017. PMID: 28666462 Free PMC article.
-
Increased Expression of Follistatin in Breast Cancer Reduces Invasiveness and Clinically Correlates with Better Survival.Cancer Genomics Proteomics. 2017 Jul-Aug;14(4):241-251. doi: 10.21873/cgp.20035. Cancer Genomics Proteomics. 2017. PMID: 28647698 Free PMC article.
-
Follistatin suppresses the production of experimental multiple-organ metastasis by small cell lung cancer cells in natural killer cell-depleted SCID mice.Clin Cancer Res. 2008 Feb 1;14(3):660-7. doi: 10.1158/1078-0432.CCR-07-1221. Clin Cancer Res. 2008. PMID: 18245525
-
When tumor suppressor TGFβ meets the HER2 (ERBB2) oncogene.J Mammary Gland Biol Neoplasia. 2011 Jun;16(2):81-8. doi: 10.1007/s10911-011-9206-4. Epub 2011 Apr 6. J Mammary Gland Biol Neoplasia. 2011. PMID: 21590373 Free PMC article. Review.
-
Oncogenic and tumor-suppressive mouse models for breast cancer engaging HER2/neu.Int J Cancer. 2017 Feb 1;140(3):495-503. doi: 10.1002/ijc.30399. Epub 2016 Sep 12. Int J Cancer. 2017. PMID: 27553713 Free PMC article. Review.
Cited by
-
The Reign of Follistatin in Tumors and Their Microenvironment: Implications for Drug Resistance.Biology (Basel). 2024 Feb 19;13(2):130. doi: 10.3390/biology13020130. Biology (Basel). 2024. PMID: 38392348 Free PMC article. Review.
-
INHBA(+) cancer-associated fibroblasts generate an immunosuppressive tumor microenvironment in ovarian cancer.NPJ Precis Oncol. 2024 Feb 15;8(1):35. doi: 10.1038/s41698-024-00523-y. NPJ Precis Oncol. 2024. PMID: 38360876 Free PMC article.
-
A SNAI2-PEAK1-INHBA stromal axis drives progression and lapatinib resistance in HER2-positive breast cancer by supporting subpopulations of tumor cells positive for antiapoptotic and stress signaling markers.Oncogene. 2021 Aug;40(33):5224-5235. doi: 10.1038/s41388-021-01906-2. Epub 2021 Jul 8. Oncogene. 2021. PMID: 34239043 Free PMC article.
-
An integrated genomic approach identifies follistatin as a target of the p63-epidermal growth factor receptor oncogenic network in head and neck squamous cell carcinoma.NAR Cancer. 2023 Jul 24;5(3):zcad038. doi: 10.1093/narcan/zcad038. eCollection 2023 Sep. NAR Cancer. 2023. PMID: 37492374 Free PMC article.
-
Down-regulated FST expression is involved in the poor prognosis of triple-negative breast cancer.Cancer Cell Int. 2021 May 17;21(1):267. doi: 10.1186/s12935-021-01977-x. Cancer Cell Int. 2021. PMID: 34001106 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

