Effect of topical beclomethasone on inflammatory markers in adults with eosinophilic esophagitis: A pilot study
- PMID: 28583232
- PMCID: PMC5468761
- DOI: 10.2500/ar.2017.8.0202
Effect of topical beclomethasone on inflammatory markers in adults with eosinophilic esophagitis: A pilot study
Abstract
Background: Topical corticosteroids have proven efficacy in the treatment of eosinophilic esophagitis (EoE) and are considered the cornerstone of therapy.
Objective: To evaluate the effect of topical beclomethasone dipropionate (BDP) therapy on clinical outcomes, esophageal eosinophilia, and other markers of inflammation in patients with EoE.
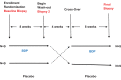
Methods: Nine subjects with a biopsy-proven diagnosis of EoE were enrolled. In a cross-over design, the subjects were randomly assigned to a sequence of BDP and placebo. Treatment periods were 8 weeks, with a 4-week washout period. The subjects had endoscopic biopsies and blood tests at baseline and after each treatment period. They were instructed to maintain a diary of symptoms. Immuno-histochemical studies were performed for interleukins IL-4, IL-5, IL-13, granulocyte-macrophage colony-stimulating factor (GM-CSF), and transforming growth factor (TGF) beta. Reverse transcription polymerase chain reaction was performed for IL-3, IL-4, IL-5, IL-10, IL-13, IL-17F, IL-25, IL-33, chemokine ligands (CCL)2, CCL5, CCL11, GM-CSF, and TGF-beta levels. The mast cell tryptase (MCT) level was measured in esophageal tissues.
Results: BDP led to a significantly larger decrease in esophageal eosinophilia compared with placebo, but there was no significant change in peripheral eosinophilia and high-sensitivity C-reactive protein between the two groups. The study was not powered enough for us to report a significant improvement in clinical symptoms. There was a significant decrease in tissue IL-13 and MCT levels from baseline to the end of treatment between the treatment and placebo groups. Mean fold decreases in cytokine expression between the baseline and treatment groups were observed for IL-17F, IL-25, CCL2, and CCL5.
Conclusion: Treatment with topical BDP was associated with significant decrease in esophageal eosinophilia, MCT and IL-13. BDP is a potential alternative to fluticasone propionate and budesonide for treatment of EoE. Larger studies are needed to validate these findings.
Conflict of interest statement
The authors have no conflicts of interest to declare pertaining to this article
Figures



References
-
- Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 133:1342–1363, 2007. - PubMed
-
- Assa'ad AH, Putnam PE, Collins MH, et al. Pediatric patients with eosinophilic esophagitis: An 8-year follow-up. J Allergy Clin Immunol 119:731–738, 2007. - PubMed
-
- Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 108:679–692; quiz 93, 2013. - PubMed
-
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol 128:3–20.e6; quiz 21–22, 2011. - PubMed
-
- Lucendo AJ, Arias Á, Molina-Infante J. Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 14:13–22.e1, 2016. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

