Proteomic composition and immunomodulatory properties of urinary bladder matrix scaffolds in homeostasis and injury
- PMID: 28583764
- PMCID: PMC8509637
- DOI: 10.1016/j.smim.2017.05.002
Proteomic composition and immunomodulatory properties of urinary bladder matrix scaffolds in homeostasis and injury
Abstract
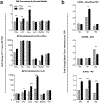
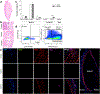
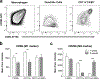
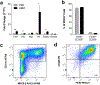
Urinary bladder matrix (UBM) is used clinically for management of wounds and reinforcement of surgical soft tissue repair, among other applications. UBM consists of the lamina propria and basal lamina of the porcine urinary bladder, and is decellularized as part of the process to manufacture the medical device. UBM is composed mainly of Collagen I, but also contains a wide variety of fibrillar and basement membrane collagens, glycoproteins, proteoglycans and ECM-associated factors. Upon application of the biomaterial in a traumatic or non-traumatic setting in a mouse model, there is a cascade of immune cells that respond to the damaged tissue and biomaterial. Here, through the use of multicolor flow cytometry, we describe the various cells that infiltrate the UBM scaffold in a subcutaneous and volumetric muscle injury model. A wide variety of immune cells are found in the UBM scaffold immune microenvironment (SIM) including F4/80+ macrophages, CD11c+ dendritic cells, CD3+ T cells and CD19+ B cells. A systemic IL-4 upregulation and a local M2-macrophage response were observed in the proximity of the implanted UBM. The recruitment and activation of these cells is dependent upon signals from the scaffold and communication between the different cell types present.
Keywords: Biomaterials; Immunology; Macrophages; T cells.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Production and Preparation of Porcine Urinary Bladder Matrix (UBM) for Urinary Bladder Tissue-Engineering Purposes.Adv Exp Med Biol. 2021;1345:119-128. doi: 10.1007/978-3-030-82735-9_10. Adv Exp Med Biol. 2021. PMID: 34582018
-
Inhibition of COX1/2 alters the host response and reduces ECM scaffold mediated constructive tissue remodeling in a rodent model of skeletal muscle injury.Acta Biomater. 2016 Feb;31:50-60. doi: 10.1016/j.actbio.2015.11.043. Epub 2015 Dec 2. Acta Biomater. 2016. PMID: 26612417 Free PMC article.
-
[Influence of porcine urinary bladder matrix and porcine acellular dermal matrix on wound healing of full-thickness skin defect in diabetic mice].Zhonghua Shao Shang Za Zhi. 2020 Dec 20;36(12):1130-1138. doi: 10.3760/cma.j.cn501120-20200901-00399. Zhonghua Shao Shang Za Zhi. 2020. PMID: 33379849 Chinese.
-
Inflammation-mediated matrix remodeling of extracellular matrix-mimicking biomaterials in tissue engineering and regenerative medicine.Acta Biomater. 2022 Oct 1;151:106-117. doi: 10.1016/j.actbio.2022.08.015. Epub 2022 Aug 13. Acta Biomater. 2022. PMID: 35970482 Review.
-
Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine.Biomaterials. 2022 Oct;289:121786. doi: 10.1016/j.biomaterials.2022.121786. Epub 2022 Sep 3. Biomaterials. 2022. PMID: 36116171 Review.
Cited by
-
Recent advances in decellularized biomaterials for wound healing.Mater Today Bio. 2023 Feb 23;19:100589. doi: 10.1016/j.mtbio.2023.100589. eCollection 2023 Apr. Mater Today Bio. 2023. PMID: 36880081 Free PMC article. Review.
-
Type 2 immunity induced by bladder extracellular matrix enhances corneal wound healing.Sci Adv. 2021 Apr 16;7(16):eabe2635. doi: 10.1126/sciadv.abe2635. Print 2021 Apr. Sci Adv. 2021. PMID: 33863719 Free PMC article.
-
3D Bioprinted Patient-Specific Extracellular Matrix Scaffolds for Soft Tissue Defects.Adv Healthc Mater. 2022 Dec;11(24):e2200866. doi: 10.1002/adhm.202200866. Epub 2022 Sep 23. Adv Healthc Mater. 2022. PMID: 36063047 Free PMC article.
-
Outcome of a novel porcine-derived UBM/SIS composite biological mesh in a rabbit vaginal defect model.Int Urogynecol J. 2023 Jul;34(7):1501-1511. doi: 10.1007/s00192-022-05400-5. Epub 2022 Nov 23. Int Urogynecol J. 2023. PMID: 36472680
-
A biologic scaffold-associated type 2 immune microenvironment inhibits tumor formation and synergizes with checkpoint immunotherapy.Sci Transl Med. 2019 Jan 30;11(477):eaat7973. doi: 10.1126/scitranslmed.aat7973. Sci Transl Med. 2019. PMID: 30700576 Free PMC article.
References
-
- Badylak SF, Freytes DO, Gilbert TW, Extracellular matrix as a biological scaffold material: structure and function, Acta Biomater. 5 (1) (2009) 1–13. - PubMed
-
- Gilbert TW, Stolz DB, Biancaniello F, Simmons-Byrd A, Badylak SF, Production and characterization of ECM powder: implications for tissue engineering applications, Biomaterials 26 (12) (2005) 1431–1435. - PubMed
-
- Gilbert TW, Sellaro TL, Badylak SF, Decellularization of tissues and organs, Biomaterials 27 (19) (2006) 3675–3683. - PubMed
-
- Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, Turner NJ, Weber DJ, Simpson TW, Wyse A, Brown EH, Dziki JL, Fisher LE, Brown S, Badylak SF, An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss, Sci. Transl. Med 6 (234) (2014) 234ra58. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

