Cyp1b1 deletion and retinol deficiency coordinately suppress mouse liver lipogenic genes and hepcidin expression during post-natal development
- PMID: 28583802
- PMCID: PMC5985816
- DOI: 10.1016/j.mce.2017.05.037
Cyp1b1 deletion and retinol deficiency coordinately suppress mouse liver lipogenic genes and hepcidin expression during post-natal development
Abstract
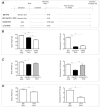
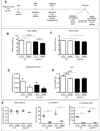
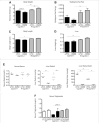
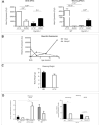
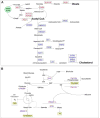
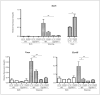
Cyp1b1 deletion and gestational vitamin A deficiency (GVAD) redirect adult liver gene expression. A matched sufficient pre- and post-natal diet, which has high carbohydrate and normal iron content (LF12), increased inflammatory gene expression markers in adult livers that were suppressed by GVAD and Cyp1b1 deletion. At birth on the LF12 diet, Cyp1b1 deletion and GVAD each suppress liver expression of the iron suppressor, hepcidin (Hepc), while increasing stellate cell activation markers and suppressing post-natal increases in lipogenesis. Hepc was less suppressed in Cyp1b1-/- pups with a standard breeder diet, but was restored by iron supplementation of the LF12 diet.
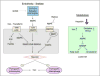
Conclusions: The LF12 diet delivered low post-natal iron and attenuated Hepc. Hepc decreases in Cyp1b1-/- and GVAD mice resulted in stellate activation and lipogenesis suppression. Endothelial BMP6, a Hepc stimulant, is a potential coordinator and Cyp1b1 target. These neonatal changes in Cyp1b1-/- mice link to diminished adult obesity and liver inflammation.
Keywords: Cytochrome P450 1b1; Hepcidin; Lipogenesis; Vitamin A; Vitamin A deficiency.
Copyright © 2017. Published by Elsevier B.V.
Figures
Similar articles
-
Cyp1b1 directs Srebp-mediated cholesterol and retinoid synthesis in perinatal liver; Association with retinoic acid activity during fetal development.PLoS One. 2020 Feb 6;15(2):e0228436. doi: 10.1371/journal.pone.0228436. eCollection 2020. PLoS One. 2020. PMID: 32027669 Free PMC article.
-
Diet-dependent retinoid effects on liver gene expression include stellate and inflammation markers and parallel effects of the nuclear repressor Shp.J Nutr Biochem. 2017 Sep;47:63-74. doi: 10.1016/j.jnutbio.2017.04.009. Epub 2017 Apr 19. J Nutr Biochem. 2017. PMID: 28570941 Free PMC article.
-
Defined Diets Link Iron and α-Linolenic Acid to Cyp1b1 Regulation of Neonatal Liver Development Through Srebp Forms and LncRNA H19.Int J Mol Sci. 2025 Feb 25;26(5):2011. doi: 10.3390/ijms26052011. Int J Mol Sci. 2025. PMID: 40076634 Free PMC article.
-
Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD).Nutrients. 2017 Dec 29;10(1):29. doi: 10.3390/nu10010029. Nutrients. 2017. PMID: 29286303 Free PMC article. Review.
-
Cytochrome P450 1B1: A Key Regulator of Ocular Iron Homeostasis and Oxidative Stress.Cells. 2022 Sep 20;11(19):2930. doi: 10.3390/cells11192930. Cells. 2022. PMID: 36230892 Free PMC article. Review.
Cited by
-
Cytochrome P4501B1 in bone marrow is co-expressed with key markers of mesenchymal stem cells. BMS2 cell line models PAH disruption of bone marrow niche development functions.Toxicol Appl Pharmacol. 2020 Aug 15;401:115111. doi: 10.1016/j.taap.2020.115111. Epub 2020 Jun 14. Toxicol Appl Pharmacol. 2020. PMID: 32553695 Free PMC article.
-
Recent insights on the role and regulation of retinoic acid signaling during epicardial development.Genesis. 2019 Jul;57(7-8):e23303. doi: 10.1002/dvg.23303. Epub 2019 May 8. Genesis. 2019. PMID: 31066193 Free PMC article. Review.
-
Time-dependent changes in retinoids content in liver and adipose tissue after feeding of a vitamin A-deficient diet to mice.Exp Anim. 2024 Jul 9;73(3):302-309. doi: 10.1538/expanim.23-0123. Epub 2024 Feb 21. Exp Anim. 2024. PMID: 38382988 Free PMC article.
-
Cyp1b1 expression impacts the angiogenic and inflammatory properties of liver sinusoidal endothelial cells.PLoS One. 2018 Oct 29;13(10):e0206756. doi: 10.1371/journal.pone.0206756. eCollection 2018. PLoS One. 2018. PMID: 30372497 Free PMC article.
-
Cyp1b1 directs Srebp-mediated cholesterol and retinoid synthesis in perinatal liver; Association with retinoic acid activity during fetal development.PLoS One. 2020 Feb 6;15(2):e0228436. doi: 10.1371/journal.pone.0228436. eCollection 2020. PLoS One. 2020. PMID: 32027669 Free PMC article.
References
-
- Armitage AE, Eddowes LA, Gileadi U, Cole S, Spottiswoode N, Selvakumar TA, Ho LP, Townsend AR, Drakesmith H. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118:4129–39. - PubMed
-
- Arndt S, Wacker E, Dorn C, Koch A, Saugspier M, Thasler WE, Hartmann A, Bosserhoff AK, Hellerbrand C. Enhanced expression of BMP6 inhibits hepatic fibrosis in non-alcoholic fatty liver disease. Gut. 2015;64:973–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

