Neutrophils Slow Disease Progression in Murine Lupus via Modulation of Autoreactive Germinal Centers
- PMID: 28584005
- PMCID: PMC5524201
- DOI: 10.4049/jimmunol.1700354
Neutrophils Slow Disease Progression in Murine Lupus via Modulation of Autoreactive Germinal Centers
Abstract
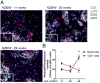
Neutrophils are well characterized as mediators of peripheral tissue damage in lupus, but it remains unclear whether they influence loss of self-tolerance in the adaptive immune compartment. Lupus neutrophils produce elevated levels of factors known to fuel autoantibody production, including IL-6 and B cell survival factors, but also reactive oxygen intermediates, which can suppress lymphocyte proliferation. To assess whether neutrophils directly influence the progression of autoreactivity in secondary lymphoid organs (SLOs), we characterized the localization and cell-cell contacts of splenic neutrophils at several stages in the progression of disease in the NZB/W murine model of lupus. Neutrophils accumulate in SLO over the course of lupus progression, preferentially localizing near T lymphocytes early in disease and B cells with advanced disease. RNA sequencing reveals that the splenic neutrophil transcriptional program changes significantly over the course of disease, with neutrophil expression of anti-inflammatory mediators peaking during early-stage and midstage disease, and evidence of neutrophil activation with advanced disease. To assess whether neutrophils exert predominantly protective or deleterious effects on loss of B cell self-tolerance in vivo, we depleted neutrophils at different stages of disease. Neutrophil depletion early in lupus resulted in a striking acceleration in the onset of renal disease, SLO germinal center formation, and autoreactive plasma cell production. In contrast, neutrophil depletion with more advanced disease did not alter systemic lupus erythematosus progression. These results demonstrate a surprising temporal and context-dependent role for neutrophils in restraining autoreactive B cell activation in lupus.
Copyright © 2017 by The American Association of Immunologists, Inc.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


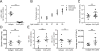



Similar articles
-
Long-term B cell depletion in murine lupus eliminates autoantibody-secreting cells and is associated with alterations in the kidney plasma cell niche.J Immunol. 2014 Apr 1;192(7):3011-20. doi: 10.4049/jimmunol.1302003. Epub 2014 Feb 26. J Immunol. 2014. PMID: 24574498 Free PMC article.
-
Thymic B Cells Promote Germinal Center-Like Structures and the Expansion of Follicular Helper T Cells in Lupus-Prone Mice.Front Immunol. 2020 Apr 28;11:696. doi: 10.3389/fimmu.2020.00696. eCollection 2020. Front Immunol. 2020. PMID: 32411134 Free PMC article.
-
Neutrophils contribute to excess serum BAFF levels and promote CD4+ T cell and B cell responses in lupus-prone mice.PLoS One. 2014 Jul 10;9(7):e102284. doi: 10.1371/journal.pone.0102284. eCollection 2014. PLoS One. 2014. PMID: 25010693 Free PMC article.
-
Dysregulated Lymphoid Cell Populations in Mouse Models of Systemic Lupus Erythematosus.Clin Rev Allergy Immunol. 2017 Oct;53(2):181-197. doi: 10.1007/s12016-017-8605-8. Clin Rev Allergy Immunol. 2017. PMID: 28500565 Review.
-
Autoreactive T cells in murine lupus: origins and roles in autoantibody production.Immunol Res. 1999;19(2-3):245-57. doi: 10.1007/BF02786492. Immunol Res. 1999. PMID: 10493178 Review.
Cited by
-
A Novel Supplementation Approach to Enhance Host Response to Sublingual Vaccination.Sci Rep. 2019 Jan 24;9(1):715. doi: 10.1038/s41598-018-36370-8. Sci Rep. 2019. PMID: 30679470 Free PMC article.
-
Androgen-Induced Immunosuppression.Front Immunol. 2018 Apr 17;9:794. doi: 10.3389/fimmu.2018.00794. eCollection 2018. Front Immunol. 2018. PMID: 29755457 Free PMC article. Review.
-
Characterization of a mouse model of osteoarticular infection using clinical isolates of Staphylococcus aureus.JBMR Plus. 2025 May 19;9(9):ziaf093. doi: 10.1093/jbmrpl/ziaf093. eCollection 2025 Sep. JBMR Plus. 2025. PMID: 40800669 Free PMC article.
-
Neutrophils in secondary lymphoid organs.Immunology. 2021 Dec;164(4):677-688. doi: 10.1111/imm.13406. Epub 2021 Aug 30. Immunology. 2021. PMID: 34411302 Free PMC article. Review.
-
Murine lupus is neutrophil elastase-independent in the MRL.Faslpr model.PLoS One. 2020 Apr 3;15(4):e0226396. doi: 10.1371/journal.pone.0226396. eCollection 2020. PLoS One. 2020. PMID: 32243431 Free PMC article.
References
-
- Smith CK, Kaplan MJ. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Current opinion in rheumatology. 2015;27:448–453. - PubMed
-
- Pradhan VD, Badakere SS, Bichile LS, Almeida AF. Anti-neutrophil cytoplasmic antibodies (ANCA) in systemic lupus erythematosus: prevalence, clinical associations and correlation with other autoantibodies. J Assoc Physicians India. 2004;52:533–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

