Conformational biosensors reveal allosteric interactions between heterodimeric AT1 angiotensin and prostaglandin F2α receptors
- PMID: 28584054
- PMCID: PMC5519365
- DOI: 10.1074/jbc.M117.793877
Conformational biosensors reveal allosteric interactions between heterodimeric AT1 angiotensin and prostaglandin F2α receptors
Abstract
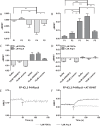
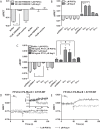
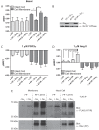
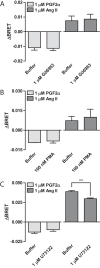
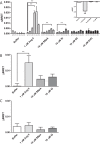
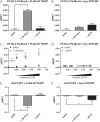
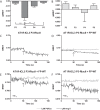
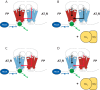
G protein-coupled receptors (GPCRs) are conformationally dynamic proteins transmitting ligand-encoded signals in multiple ways. This transmission is highly complex and achieved through induction of distinct GPCR conformations, which preferentially drive specific receptor-mediated signaling events. This conformational capacity can be further enlarged via allosteric effects between dimers, warranting further study of these effects. Using GPCR conformation-sensitive biosensors, we investigated allosterically induced conformational changes in the recently reported F prostanoid (FP)/angiotensin II type 1 receptor (AT1R) heterodimer. Ligand occupancy of the AT1R induced distinct conformational changes in FP compared with those driven by PGF2α in bioluminescence resonance energy transfer (BRET)-based FP biosensors engineered with Renilla luciferase (RLuc) as an energy donor in the C-tail and fluorescein arsenical hairpin binder (FlAsH)-labeled acceptors at different positions in the intracellular loops. We also found that this allosteric communication is mediated through Gαq and may also involve proximal (phospholipase C) but not distal (protein kinase C) signaling partners. Interestingly, β-arrestin-biased AT1R agonists could also transmit a Gαq-dependent signal to FP without activation of downstream Gαq signaling. This transmission of information was specific to the AT1R/FP complex, as activation of Gαq by the oxytocin receptor did not recapitulate the same phenomenon. Finally, information flow was asymmetric in the sense that FP activation had negligible effects on AT1R-based conformational biosensors. The identification of partner-induced GPCR conformations may help identify novel allosteric effects when investigating multiprotein receptor signaling complexes.
Keywords: G protein-coupled receptor (GPCR); bioluminescence resonance energy transfer (BRET); cell signaling; conformational change; dimerization.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Similar articles
-
Prostaglandin F2α and angiotensin II type 1 receptors exhibit differential cognate G protein coupling regulation.J Biol Chem. 2022 Sep;298(9):102294. doi: 10.1016/j.jbc.2022.102294. Epub 2022 Jul 21. J Biol Chem. 2022. PMID: 35872018 Free PMC article.
-
Allosteric modulation of β-arrestin-biased angiotensin II type 1 receptor signaling by membrane stretch.J Biol Chem. 2014 Oct 10;289(41):28271-83. doi: 10.1074/jbc.M114.585067. Epub 2014 Aug 28. J Biol Chem. 2014. PMID: 25170081 Free PMC article.
-
Angiotensin II type I and prostaglandin F2α receptors cooperatively modulate signaling in vascular smooth muscle cells.J Biol Chem. 2015 Jan 30;290(5):3137-48. doi: 10.1074/jbc.M114.631119. Epub 2014 Dec 15. J Biol Chem. 2015. PMID: 25512374 Free PMC article.
-
Exploring functional consequences of GPCR oligomerization requires a different lens.Prog Mol Biol Transl Sci. 2020;169:181-211. doi: 10.1016/bs.pmbts.2019.11.001. Epub 2019 Dec 10. Prog Mol Biol Transl Sci. 2020. PMID: 31952686 Review.
-
Current topics in angiotensin II type 1 receptor research: Focus on inverse agonism, receptor dimerization and biased agonism.Pharmacol Res. 2017 Sep;123:40-50. doi: 10.1016/j.phrs.2017.06.013. Epub 2017 Jun 23. Pharmacol Res. 2017. PMID: 28648738 Free PMC article. Review.
Cited by
-
Finding the Perfect Fit: Conformational Biosensors to Determine the Efficacy of GPCR Ligands.ACS Pharmacol Transl Sci. 2022 Aug 14;5(9):694-709. doi: 10.1021/acsptsci.1c00256. eCollection 2022 Sep 9. ACS Pharmacol Transl Sci. 2022. PMID: 36110374 Free PMC article. Review.
-
Addition of a carboxy-terminal tail to the normally tailless gonadotropin-releasing hormone receptor impairs fertility in female mice.Elife. 2021 Dec 23;10:e72937. doi: 10.7554/eLife.72937. Elife. 2021. PMID: 34939930 Free PMC article.
-
Activation of 2-oxoglutarate receptor 1 (OXGR1) by α-ketoglutarate (αKG) does not detectably stimulate Pendrin-mediated anion exchange in Xenopus oocytes.Physiol Rep. 2022 Jul;10(14):e15362. doi: 10.14814/phy2.15362. Physiol Rep. 2022. PMID: 35851763 Free PMC article.
-
Prostaglandin F2α requires activation of calcium-dependent signalling to trigger inflammation in human myometrium.Front Endocrinol (Lausanne). 2023 Jul 19;14:1150125. doi: 10.3389/fendo.2023.1150125. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37547305 Free PMC article.
-
Pharmacology of Free Fatty Acid Receptors and Their Allosteric Modulators.Int J Mol Sci. 2021 Feb 10;22(4):1763. doi: 10.3390/ijms22041763. Int J Mol Sci. 2021. PMID: 33578942 Free PMC article. Review.
References
-
- Lane J. R., Donthamsetti P., Shonberg J., Draper-Joyce C. J., Dentry S., Michino M., Shi L., López L., Scammells P. J., Capuano B., Sexton P. M., Javitch J. A., and Christopoulos A. (2014) A new mechanism of allostery in a G protein-coupled receptor dimer. Nat. Chem. Biol. 10, 745–752 - PMC - PubMed
-
- Rasmussen S. G., DeVree B. T., Zou Y., Kruse A. C., Chung K. Y., Kobilka T. S., Thian F. S., Chae P. S., Pardon E., Calinski D., Mathiesen J. M., Shah S. T., Lyons J. A., Caffrey M., Gellman S. H., et al. (2011) Crystal structure of the β2-adrenergic receptor-Gs protein complex. Nature 477, 549–555 - PMC - PubMed
-
- Huang W., Manglik A., Venkatakrishnan A. J., Laeremans T., Feinberg E. N., Sanborn A. L., Kato H. E., Livingston K. E., Thorsen T. S., Kling R. C., Granier S., Gmeiner P., Husbands S. M., Traynor J. R., Weis W. I., et al. (2015) Structural insights into μ-opioid receptor activation. Nature 524, 315–321 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

