Cytochrome b5 Reductase 3 Modulates Soluble Guanylate Cyclase Redox State and cGMP Signaling
- PMID: 28584062
- PMCID: PMC5527687
- DOI: 10.1161/CIRCRESAHA.117.310705
Cytochrome b5 Reductase 3 Modulates Soluble Guanylate Cyclase Redox State and cGMP Signaling
Abstract
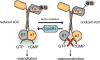
Rationale: Soluble guanylate cyclase (sGC) heme iron, in its oxidized state (Fe3+), is desensitized to NO and limits cGMP production needed for downstream activation of protein kinase G-dependent signaling and blood vessel dilation.
Objective: Although reactive oxygen species are known to oxidize the sGC heme iron, the basic mechanism(s) governing sGC heme iron recycling to its NO-sensitive, reduced state remain poorly understood.
Methods and results: Oxidant challenge studies show that vascular smooth muscle cells have an intrinsic ability to reduce oxidized sGC heme iron and form protein-protein complexes between cytochrome b5 reductase 3, also known as methemoglobin reductase, and oxidized sGC. Genetic knockdown and pharmacological inhibition in vascular smooth muscle cells reveal that cytochrome b5 reductase 3 expression and activity is critical for NO-stimulated cGMP production and vasodilation. Mechanistically, we show that cytochrome b5 reductase 3 directly reduces oxidized sGC required for NO sensitization as assessed by biochemical, cellular, and ex vivo assays.
Conclusions: Together, these findings identify new insights into NO-sGC-cGMP signaling and reveal cytochrome b5 reductase 3 as the first identified physiological sGC heme iron reductase in vascular smooth muscle cells, serving as a critical regulator of cGMP production and protein kinase G-dependent signaling.
Keywords: guanosine; heme; iron; nitric oxide; reactive oxygen species.
© 2017 American Heart Association, Inc.
Conflict of interest statement
The authors declare no conflicts of interest with the contents of this article.
Figures






References
-
- Zhao Y, Marletta MA. Localization of the heme binding region in soluble guanylate cyclase. Biochemistry. 1997;36:15959–15964. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

