Application of Zone Model Predictive Control Artificial Pancreas During Extended Use of Infusion Set and Sensor: A Randomized Crossover-Controlled Home-Use Trial
- PMID: 28584075
- PMCID: PMC5521973
- DOI: 10.2337/dc17-0500
Application of Zone Model Predictive Control Artificial Pancreas During Extended Use of Infusion Set and Sensor: A Randomized Crossover-Controlled Home-Use Trial
Erratum in
-
Erratum. Application of Zone Model Predictive Control Artificial Pancreas During Extended Use of Infusion Set and Sensor: A Randomized Crossover-Controlled Home-Use Trial. Diabetes Care 2017;40:1096-1102.Diabetes Care. 2017 Nov;40(11):1606. doi: 10.2337/dc17-er11a. Epub 2017 Sep 8. Diabetes Care. 2017. PMID: 28887408 Free PMC article. No abstract available.
Abstract
Objective: As artificial pancreas (AP) becomes standard of care, consideration of extended use of insulin infusion sets (IIS) and continuous glucose monitors (CGMs) becomes vital. We conducted an outpatient randomized crossover study to test the safety and efficacy of a zone model predictive control (zone-MPC)-based AP system versus sensor augmented pump (SAP) therapy in which IIS and CGM failures were provoked via extended wear to 7 and 21 days, respectively.
Research design and methods: A smartphone-based AP system was used by 19 adults (median age 23 years [IQR 10], mean 8.0 ± 1.7% HbA1c) over 2 weeks and compared with SAP therapy for 2 weeks in a crossover, unblinded outpatient study with remote monitoring in both study arms.
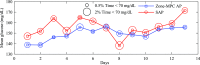
Results: AP improved percent time 70-140 mg/dL (48.1 vs. 39.2%; P = 0.016) and time 70-180 mg/dL (71.6 vs. 65.2%; P = 0.008) and decreased median glucose (141 vs. 153 mg/dL; P = 0.036) and glycemic variability (SD 52 vs. 55 mg/dL; P = 0.044) while decreasing percent time <70 mg/dL (1.3 vs. 2.7%; P = 0.001). AP also improved overnight control, as measured by mean glucose at 0600 h (140 vs. 158 mg/dL; P = 0.02). IIS failures (1.26 ± 1.44 vs. 0.78 ± 0.78 events; P = 0.13) and sensor failures (0.84 ± 0.6 vs. 1.1 ± 0.73 events; P = 0.25) were similar between AP and SAP arms. Higher percent time in closed loop was associated with better glycemic outcomes.
Conclusions: Zone-MPC significantly and safely improved glycemic control in a home-use environment despite prolonged CGM and IIS wear. This project represents the first home-use AP study attempting to provoke and detect component failure while successfully maintaining safety and effective glucose control.
Trial registration: ClinicalTrials.gov NCT02773875.
© 2017 by the American Diabetes Association.
Figures


Similar articles
-
2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial.Lancet Diabetes Endocrinol. 2015 Dec;3(12):939-47. doi: 10.1016/S2213-8587(15)00335-6. Epub 2015 Sep 30. Lancet Diabetes Endocrinol. 2015. PMID: 26432775 Clinical Trial.
-
Day-and-Night Closed-Loop Glucose Control in Patients With Type 1 Diabetes Under Free-Living Conditions: Results of a Single-Arm 1-Month Experience Compared With a Previously Reported Feasibility Study of Evening and Night at Home.Diabetes Care. 2016 Jul;39(7):1151-60. doi: 10.2337/dc16-0008. Epub 2016 May 5. Diabetes Care. 2016. PMID: 27208331 Clinical Trial.
-
Glycemia, Treatment Satisfaction, Cognition, and Sleep Quality in Adults and Adolescents with Type 1 Diabetes When Using a Closed-Loop System Overnight Versus Sensor-Augmented Pump with Low-Glucose Suspend Function: A Randomized Crossover Study.Diabetes Technol Ther. 2016 Dec;18(12):772-783. doi: 10.1089/dia.2016.0288. Epub 2016 Nov 11. Diabetes Technol Ther. 2016. PMID: 27835037 Clinical Trial.
-
The International Diabetes Closed-Loop Study: Testing Artificial Pancreas Component Interoperability.Diabetes Technol Ther. 2019 Feb;21(2):73-80. doi: 10.1089/dia.2018.0308. Epub 2019 Jan 16. Diabetes Technol Ther. 2019. PMID: 30649925 Free PMC article.
-
Diabetes technology and treatments in the paediatric age group.Int J Clin Pract Suppl. 2011 Feb;(170):76-82. doi: 10.1111/j.1742-1241.2010.02582.x. Int J Clin Pract Suppl. 2011. PMID: 21323816 Review.
Cited by
-
Controlling the AP Controller: Controller Performance Assessment and Modification.J Diabetes Sci Technol. 2019 Nov;13(6):1091-1104. doi: 10.1177/1932296819877217. Epub 2019 Sep 27. J Diabetes Sci Technol. 2019. PMID: 31561714 Free PMC article.
-
Velocity-weighting & velocity-penalty MPC of an artificial pancreas: Improved safety & performance.Automatica (Oxf). 2018 May;91:105-117. doi: 10.1016/j.automatica.2018.01.025. Epub 2018 Mar 20. Automatica (Oxf). 2018. PMID: 30034017 Free PMC article.
-
Artificial Pancreas: Current Progress and Future Outlook in the Treatment of Type 1 Diabetes.Drugs. 2019 Jul;79(10):1089-1101. doi: 10.1007/s40265-019-01149-2. Drugs. 2019. PMID: 31190305 Review.
-
The UVA/Padova Type 1 Diabetes Simulator Goes From Single Meal to Single Day.J Diabetes Sci Technol. 2018 Mar;12(2):273-281. doi: 10.1177/1932296818757747. Epub 2018 Feb 16. J Diabetes Sci Technol. 2018. PMID: 29451021 Free PMC article.
-
International Society for Pediatric and Adolescent Diabetes Clinical Practice Consensus Guidelines 2024: Diabetes Technologies - Insulin Delivery.Horm Res Paediatr. 2024;97(6):636-662. doi: 10.1159/000543034. Epub 2024 Dec 10. Horm Res Paediatr. 2024. PMID: 39657603 Free PMC article. Review.
References
-
- Kowalski A. Pathway to artificial pancreas systems revisited: moving downstream. Diabetes Care 2015;38:1036–1043 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

