Frontline Science: Aspirin-triggered resolvin D1 controls herpes simplex virus-induced corneal immunopathology
- PMID: 28584076
- PMCID: PMC5636045
- DOI: 10.1189/jlb.3HI1216-511RR
Frontline Science: Aspirin-triggered resolvin D1 controls herpes simplex virus-induced corneal immunopathology
Abstract
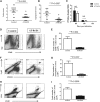
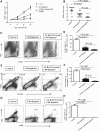
Stromal keratitis (SK) is a chronic immunopathological lesion of the eye, caused by HSV-1 infection, and a common cause of vision impairment in humans. The inflammatory lesions in the cornea are primarily caused by neutrophils with the active participation of CD4+ T cells. Therefore, the targeting of these immune cell types and their products represents a potentially valuable form of therapy to reduce the severity of disease. Resolvin D1 (RvD1) and its epimer aspirin-triggered RvD1 (AT-RvD1) are lipid mediators derived from docosahexaenoic acid (DHA) and were shown to promote resolution in several inflammatory disease models. In this report, we examined whether AT-RvD1 administration, begun before infection or at a later stage after ocular infection of mice with HSV-1, could control the severity of SK lesions. Treatment with AT-RvD1 significantly diminished the extent of corneal neovascularization and the severity of SK lesions. AT-RvD1-treated mice had fewer numbers of inflammatory cells that included neutrophils as well as Th1 and Th17 cells in the infected cornea. The mechanisms by which AT-RvD1 acts appear to be multiple. These include inhibitory effects on proinflammatory mediators, such as IL-1β, IL-6, IL-12, CXCL1, MCP-1, MIP-2, vascular endothelial growth factor (VEGF)-A, matrix metalloproteinase 9 (MMP-9), and proinflammatory miRNA, such as miR-155, miR-132, and miR-223, which are involved in SK pathogenesis and corneal neovascularization. In addition, AT-RvD1 attenuated STAT1, which plays an important role in Th1 cell differentiation and IFN-γ expression. These findings demonstrate that AT-RvD1 treatment could represent a useful strategy for the management of virus-induced immunopathological lesions.
Keywords: inflammation; resolvins; stromal keratitis.
© Society for Leukocyte Biology.
Figures






Comment in
-
Editorial: Resolving herpes-induced, ocular pathology: can fish oil really do that?J Leukoc Biol. 2017 Nov;102(5):1153-1155. doi: 10.1189/jlb.3CE0617-236R. J Leukoc Biol. 2017. PMID: 29093132 No abstract available.
Similar articles
-
Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1.J Immunol. 2011 Feb 1;186(3):1735-46. doi: 10.4049/jimmunol.1003456. Epub 2010 Dec 27. J Immunol. 2011. PMID: 21187448 Free PMC article.
-
Role of miR-155 in the pathogenesis of herpetic stromal keratitis.Am J Pathol. 2015 Apr;185(4):1073-84. doi: 10.1016/j.ajpath.2014.12.021. Epub 2015 Feb 18. Am J Pathol. 2015. PMID: 25700796 Free PMC article.
-
Galectin-1 reduces the severity of herpes simplex virus-induced ocular immunopathological lesions.J Immunol. 2012 May 1;188(9):4631-43. doi: 10.4049/jimmunol.1103063. Epub 2012 Mar 30. J Immunol. 2012. PMID: 22467659 Free PMC article.
-
Pathogenesis of Herpes Stromal Keratitis: Immune Inflammatory Response Mediated by Inflammatory Regulators.Front Immunol. 2020 May 13;11:766. doi: 10.3389/fimmu.2020.00766. eCollection 2020. Front Immunol. 2020. PMID: 32477330 Free PMC article. Review.
-
Application of our understanding of pathogenesis of herpetic stromal keratitis for novel therapy.Microbes Infect. 2018 Oct-Nov;20(9-10):526-530. doi: 10.1016/j.micinf.2017.12.014. Epub 2018 Jan 9. Microbes Infect. 2018. PMID: 29329934 Free PMC article. Review.
Cited by
-
MicroRNA-155: A Master Regulator of Inflammation.J Interferon Cytokine Res. 2019 Jun;39(6):321-330. doi: 10.1089/jir.2018.0155. Epub 2019 Mar 20. J Interferon Cytokine Res. 2019. PMID: 30998423 Free PMC article. Review.
-
Pro-resolving lipid mediators: regulators of inflammation, metabolism and kidney function.Nat Rev Nephrol. 2021 Nov;17(11):725-739. doi: 10.1038/s41581-021-00454-y. Epub 2021 Jul 19. Nat Rev Nephrol. 2021. PMID: 34282342 Free PMC article. Review.
-
MicroRNA155 Expression in Relation to BDCAF Scored Behçet's Disease in an Egyptian Patients' Sample.Open Rheumatol J. 2018 Jul 31;12:115-122. doi: 10.2174/1874312901812010115. eCollection 2018. Open Rheumatol J. 2018. PMID: 30197703 Free PMC article.
-
VIP modulates the ALX/FPR2 receptor axis toward inflammation resolution in a mouse model of bacterial keratitis.Prostaglandins Other Lipid Mediat. 2019 Feb;140:18-25. doi: 10.1016/j.prostaglandins.2018.12.001. Epub 2018 Dec 4. Prostaglandins Other Lipid Mediat. 2019. PMID: 30529189 Free PMC article. Review.
-
Eicosanoid and Specialized Proresolving Mediator Regulation of Lymphoid Cells.Trends Biochem Sci. 2019 Mar;44(3):214-225. doi: 10.1016/j.tibs.2018.10.007. Epub 2018 Nov 23. Trends Biochem Sci. 2019. PMID: 30477730 Free PMC article. Review.
References
-
- Liesegang T. J. (2001) Herpes simplex virus epidemiology and ocular importance. Cornea 20, 1–13. - PubMed
-
- Deshpande S., Banerjee K., Biswas P. S., Rouse B. T. (2004) Herpetic eye disease: immunopathogenesis and therapeutic measures. Expert Rev. Mol. Med. 6, 1–14. - PubMed
-
- McGhee C. N., Dean S., Danesh-Meyer H. (2002) Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 25, 33–55. - PubMed
-
- Knickelbein J. E., Hendricks R. L., Charukamnoetkanok P. (2009) Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv. Ophthalmol. 54, 226–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

