Whole-organism cellular gene-expression atlas reveals conserved cell types in the ventral nerve cord of Platynereis dumerilii
- PMID: 28584082
- PMCID: PMC5468599
- DOI: 10.1073/pnas.1610602114
Whole-organism cellular gene-expression atlas reveals conserved cell types in the ventral nerve cord of Platynereis dumerilii
Abstract
The comparative study of cell types is a powerful approach toward deciphering animal evolution. To avoid selection biases, however, comparisons ideally involve all cell types present in a multicellular organism. Here, we use image registration and a newly developed "Profiling by Signal Probability Mapping" algorithm to generate a cellular resolution 3D expression atlas for an entire animal. We investigate three-segmented young worms of the marine annelid Platynereis dumerilii, with a rich diversity of differentiated cells present in relatively low number. Starting from whole-mount expression images for close to 100 neural specification and differentiation genes, our atlas identifies and molecularly characterizes 605 bilateral pairs of neurons at specific locations in the ventral nerve cord. Among these pairs, we identify sets of neurons expressing similar combinations of transcription factors, located at spatially coherent anterior-posterior, dorsal-ventral, and medial-lateral coordinates that we interpret as cell types. Comparison with motor and interneuron types in the vertebrate neural tube indicates conserved combinations, for example, of cell types cospecified by Gata1/2/3 and Tal transcription factors. These include V2b interneurons and the central spinal fluid-contacting Kolmer-Agduhr cells in the vertebrates, and several neuron types in the intermediate ventral ganglionic mass in the annelid. We propose that Kolmer-Agduhr cell-like mechanosensory neurons formed part of the mucociliary sole in protostome-deuterostome ancestors and diversified independently into several neuron types in annelid and vertebrate descendants.
Keywords: Kolmer-Agduhr cells; ProSPr; cell-type evolution; evo-devo; gene-expression atlas.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



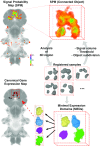















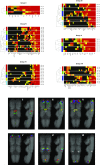



References
-
- Arendt D. The evolution of cell types in animals: Emerging principles from molecular studies. Nat Rev Genet. 2008;9:868–882. - PubMed
-
- Arendt D, et al. The origin and evolution of cell types. Nat Rev Genet. 2016;17:744–757. - PubMed
-
- Tessmar-Raible K, et al. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell. 2007;129:1389–1400. - PubMed
-
- Jacobs DK, et al. Evolution of sensory structures in basal metazoa. Integr Comp Biol. 2007;47:712–723. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

