Catalytic-site design for inverse heavy-enzyme isotope effects in human purine nucleoside phosphorylase
- PMID: 28584087
- PMCID: PMC5488955
- DOI: 10.1073/pnas.1704786114
Catalytic-site design for inverse heavy-enzyme isotope effects in human purine nucleoside phosphorylase
Abstract
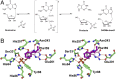
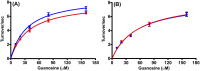
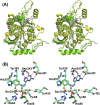
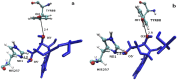
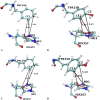
Heavy-enzyme isotope effects (15N-, 13C-, and 2H-labeled protein) explore mass-dependent vibrational modes linked to catalysis. Transition path-sampling (TPS) calculations have predicted femtosecond dynamic coupling at the catalytic site of human purine nucleoside phosphorylase (PNP). Coupling is observed in heavy PNPs, where slowed barrier crossing caused a normal heavy-enzyme isotope effect (kchemlight/kchemheavy > 1.0). We used TPS to design mutant F159Y PNP, predicted to improve barrier crossing for heavy F159Y PNP, an attempt to generate a rare inverse heavy-enzyme isotope effect (kchemlight/kchemheavy < 1.0). Steady-state kinetic comparison of light and heavy native PNPs to light and heavy F159Y PNPs revealed similar kinetic properties. Pre-steady-state chemistry was slowed 32-fold in F159Y PNP. Pre-steady-state chemistry compared heavy and light native and F159Y PNPs and found a normal heavy-enzyme isotope effect of 1.31 for native PNP and an inverse effect of 0.75 for F159Y PNP. Increased isotopic mass in F159Y PNP causes more efficient transition state formation. Independent validation of the inverse isotope effect for heavy F159Y PNP came from commitment to catalysis experiments. Most heavy enzymes demonstrate normal heavy-enzyme isotope effects, and F159Y PNP is a rare example of an inverse effect. Crystal structures and TPS dynamics of native and F159Y PNPs explore the catalytic-site geometry associated with these catalytic changes. Experimental validation of TPS predictions for barrier crossing establishes the connection of rapid protein dynamics and vibrational coupling to enzymatic transition state passage.
Keywords: enzyme design; femtosecond dynamics; heavy enzyme; purine nucleoside phosphorylase; transition path sampling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Inverse enzyme isotope effects in human purine nucleoside phosphorylase with heavy asparagine labels.Proc Natl Acad Sci U S A. 2018 Jul 3;115(27):E6209-E6216. doi: 10.1073/pnas.1805416115. Epub 2018 Jun 18. Proc Natl Acad Sci U S A. 2018. PMID: 29915028 Free PMC article.
-
Isotope-specific and amino acid-specific heavy atom substitutions alter barrier crossing in human purine nucleoside phosphorylase.Proc Natl Acad Sci U S A. 2015 Sep 8;112(36):11247-51. doi: 10.1073/pnas.1513956112. Epub 2015 Aug 24. Proc Natl Acad Sci U S A. 2015. PMID: 26305965 Free PMC article.
-
Femtosecond dynamics coupled to chemical barrier crossing in a Born-Oppenheimer enzyme.Proc Natl Acad Sci U S A. 2011 Nov 15;108(46):18661-5. doi: 10.1073/pnas.1114900108. Epub 2011 Nov 7. Proc Natl Acad Sci U S A. 2011. PMID: 22065757 Free PMC article.
-
Transition states and inhibitors of the purine nucleoside phosphorylase family.Curr Top Med Chem. 2005;5(13):1237-58. doi: 10.2174/156802605774463088. Curr Top Med Chem. 2005. PMID: 16305529 Review.
-
Promoting Vibrations and the Function of Enzymes. Emerging Theoretical and Experimental Convergence.Biochemistry. 2018 Jun 19;57(24):3299-3308. doi: 10.1021/acs.biochem.8b00201. Epub 2018 Apr 10. Biochemistry. 2018. PMID: 29608286 Free PMC article. Review.
Cited by
-
Active-Site Glu165 Activation in Triosephosphate Isomerase and Its Deprotonation Kinetics.J Phys Chem B. 2019 May 16;123(19):4230-4241. doi: 10.1021/acs.jpcb.9b02981. Epub 2019 May 2. J Phys Chem B. 2019. PMID: 31013084 Free PMC article.
-
Machine Learning Identifies Chemical Characteristics That Promote Enzyme Catalysis.J Am Chem Soc. 2019 Mar 6;141(9):4108-4118. doi: 10.1021/jacs.8b13879. Epub 2019 Feb 21. J Am Chem Soc. 2019. PMID: 30761897 Free PMC article.
-
Heavy Enzymes and the Rational Redesign of Protein Catalysts.Chembiochem. 2019 Nov 18;20(22):2807-2812. doi: 10.1002/cbic.201900134. Epub 2019 Jul 24. Chembiochem. 2019. PMID: 31016852 Free PMC article.
-
Origins of Enzyme Catalysis: Experimental Findings for C-H Activation, New Models, and Their Relevance to Prevailing Theoretical Constructs.J Am Chem Soc. 2017 Dec 27;139(51):18409-18427. doi: 10.1021/jacs.7b08418. Epub 2017 Dec 15. J Am Chem Soc. 2017. PMID: 29244501 Free PMC article.
-
Protein Mass-Modulated Effects in Alkaline Phosphatase.Biochemistry. 2021 Jan 19;60(2):118-124. doi: 10.1021/acs.biochem.0c00917. Epub 2021 Jan 7. Biochemistry. 2021. PMID: 33410323 Free PMC article.
References
-
- Núñez S, Wing C, Antoniou D, Schramm VL, Schwartz SD. Insight into catalytically relevant correlated motions in human purine nucleoside phosphorylase. J Phys Chem A. 2006;110:463–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

