Dynamic changes in murine forebrain miR-211 expression associate with cholinergic imbalances and epileptiform activity
- PMID: 28584127
- PMCID: PMC5488936
- DOI: 10.1073/pnas.1701201114
Dynamic changes in murine forebrain miR-211 expression associate with cholinergic imbalances and epileptiform activity
Abstract
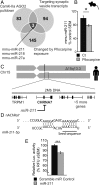
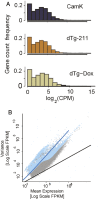
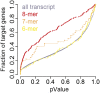
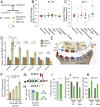
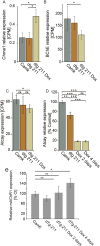
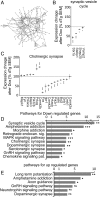
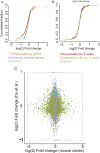
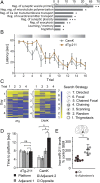
Epilepsy is a common neurological disease, manifested in unprovoked recurrent seizures. Epileptogenesis may develop due to genetic or pharmacological origins or following injury, but it remains unclear how the unaffected brain escapes this susceptibility to seizures. Here, we report that dynamic changes in forebrain microRNA (miR)-211 in the mouse brain shift the threshold for spontaneous and pharmacologically induced seizures alongside changes in the cholinergic pathway genes, implicating this miR in the avoidance of seizures. We identified miR-211 as a putative attenuator of cholinergic-mediated seizures by intersecting forebrain miR profiles that were Argonaute precipitated, synaptic vesicle target enriched, or differentially expressed under pilocarpine-induced seizures, and validated TGFBR2 and the nicotinic antiinflammatory acetylcholine receptor nAChRa7 as murine and human miR-211 targets, respectively. To explore the link between miR-211 and epilepsy, we engineered dTg-211 mice with doxycycline-suppressible forebrain overexpression of miR-211. These mice reacted to doxycycline exposure by spontaneous electrocorticography-documented nonconvulsive seizures, accompanied by forebrain accumulation of the convulsive seizures mediating miR-134. RNA sequencing demonstrated in doxycycline-treated dTg-211 cortices overrepresentation of synaptic activity, Ca2+ transmembrane transport, TGFBR2 signaling, and cholinergic synapse pathways. Additionally, a cholinergic dysregulated mouse model overexpressing a miR refractory acetylcholinesterase-R splice variant showed a parallel propensity for convulsions, miR-211 decreases, and miR-134 elevation. Our findings demonstrate that in mice, dynamic miR-211 decreases induce hypersynchronization and nonconvulsive and convulsive seizures, accompanied by expression changes in cholinergic and TGFBR2 pathways as well as in miR-134. Realizing the importance of miR-211 dynamics opens new venues for translational diagnosis of and interference with epilepsy.
Keywords: EEG; acetylcholinesterase; cholinergic; epilepsy; microRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Moshé SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: New advances. Lancet. 2015;385:884–898. - PubMed
-
- Pitkänen A, et al. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 2016;15:843–856. - PubMed
-
- Pitkänen A, Roivainen R, Lukasiuk K. 2015. Development of epilepsy after ischaemic stroke. Lancet Neurol, 10.1016/S1474-4422(15)00248-3.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

