Multimechanistic Monoclonal Antibodies (MAbs) Targeting Staphylococcus aureus Alpha-Toxin and Clumping Factor A: Activity and Efficacy Comparisons of a MAb Combination and an Engineered Bispecific Antibody Approach
- PMID: 28584141
- PMCID: PMC5527613
- DOI: 10.1128/AAC.00629-17
Multimechanistic Monoclonal Antibodies (MAbs) Targeting Staphylococcus aureus Alpha-Toxin and Clumping Factor A: Activity and Efficacy Comparisons of a MAb Combination and an Engineered Bispecific Antibody Approach
Abstract
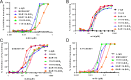
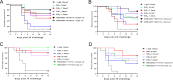
Secreted alpha-toxin and surface-localized clumping factor A (ClfA) are key virulence determinants in Staphylococcus aureus bloodstream infections. We previously demonstrated that prophylaxis with a multimechanistic monoclonal antibody (MAb) combination against alpha-toxin (MEDI4893*) and ClfA (11H10) provided greater strain coverage and improved efficacy in an S. aureus lethal bacteremia model. Subsequently, 11H10 was found to exhibit reduced affinity and impaired inhibition of fibrinogen binding to ClfA002 expressed by members of a predominant hospital-associated methicillin-resistant S. aureus (MRSA) clone, ST5. Consequently, we identified another anti-ClfA MAb (SAR114) from human tonsillar B cells with >100-fold increased affinity for three prominent ClfA variants, including ClfA002, and potent inhibition of bacterial agglutination by 112 diverse clinical isolates. We next constructed bispecific Abs (BiSAbs) comprised of 11H10 or SAR114 as IgG scaffolds and grafted anti-alpha-toxin (MEDI4893*) single-chain variable fragment to the amino or carboxy terminus of the anti-ClfA heavy chains. Although the BiSAbs exhibited in vitro potencies similar to those of the parental MAbs, only 11H10-BiSAb, but not SAR114-BiSAb, showed protective activity in murine infection models comparable to the respective MAb combination. In vivo activity with SAR114-BiSAb was observed in infection models with S. aureus lacking ClfA. Our data suggest that high-affinity binding to ClfA sequesters the SAR114-BiSAb to the bacterial surface, thereby reducing both alpha-toxin neutralization and protection in vivo These results indicate that a MAb combination targeting ClfA and alpha-toxin is more promising for future development than the corresponding BiSAb.
Keywords: Staphylococcus aureus; alpha toxin; clumping factor A; monoclonal antibodies.
Copyright © 2017 Tkaczyk et al.
Figures




References
-
- U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013....
-
- Hazenbos WL, Kajihara KK, Vandlen R, Morisaki JH, Lehar SM, Kwakkenbos MJ, Beaumont T, Bakker AQ, Phung Q, Swem LR, Ramakrishnan S, Kim J, Xu M, Shah IM, Diep BA, Sai T, Sebrell A, Khalfin Y, Oh A, Koth C, Lin SJ, Lee BC, Strandh M, Koefoed K, Andersen PS, Spits H, Brown EJ, Tan MW, Mariathasan S. 2013. Novel staphylococcal glycosyltransferases SdgA and SdgB mediate immunogenicity and protection of virulence-associated cell wall proteins. PLoS Pathog 9:e1003653. doi:10.1371/journal.ppat.1003653. - DOI - PMC - PubMed
-
- Rouha H, Badarau A, Visram ZC, Battles MB, Prinz B, Magyarics Z, Nagy G, Mirkina I, Stulik L, Zerbs M, Jagerhofer M, Maierhofer B, Teubenbacher A, Dolezilkova I, Gross K, Banerjee S, Zauner G, Malafa S, Zmajkovic J, Maier S, Mabry R, Krauland E, Wittrup KD, Gerngross TU, Nagy E. 2015. Five birds, one stone: neutralization of alpha-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs 7:243–254. doi:10.4161/19420862.2014.985132. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

