Chlamydia muridarum with Mutations in Chromosomal Genes tc0237 and/or tc0668 Is Deficient in Colonizing the Mouse Gastrointestinal Tract
- PMID: 28584162
- PMCID: PMC5520443
- DOI: 10.1128/IAI.00321-17
Chlamydia muridarum with Mutations in Chromosomal Genes tc0237 and/or tc0668 Is Deficient in Colonizing the Mouse Gastrointestinal Tract
Abstract
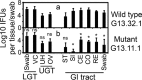
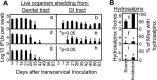
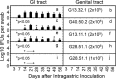
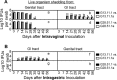
Chlamydiae colonize the gastrointestinal tracts of both animals and humans. However, their medical significance remains unknown. We have previously shown that wild-type Chlamydia muridarum spreads to and establishes stable colonization of the gastrointestinal tract following intravaginal inoculation. In the present study, we found that C. muridarum with mutations in chromosomal genes tc0237 and/or tc0668 was defective in spreading to the mouse gastrointestinal tract, which correlated with its attenuated pathogenicity in the upper genital tract. This correlation was more consistent than that of chlamydial pathogenicity with ascending infection in the genital tract, since attenuated C. muridarum spread significantly less to the gastrointestinal tract but maintained robust ascending infection of the upper genital tract. Transcervical inoculation further confirmed the correlation between C. muridarum spreading to the gastrointestinal tract and its pathogenicity in the upper genital tract. Finally, defective spreading of C. muridarum mutants was due to their inability to colonize the gastrointestinal tract since intragastric inoculation did not rescue the mutants' colonization. Thus, promoting C. muridarum colonization of the gastrointestinal tract may represent a primary function of the TC0237 and TC0668 proteins. Correlation of chlamydial colonization of the gastrointestinal tract with chlamydial pathogenicity in the upper genital tract suggests a potential role for gastrointestinal chlamydiae in genital tract pathogenicity.
Keywords: Chlamydia muridarum; TC0237; TC0668; attenuation; chlamydia; gut colonization; hydrosalpinx; mutants; mutations in tc0668 or tc0237; pathogenicity.
Copyright © 2017 American Society for Microbiology.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

