SH3 Domain-Containing Protein 2 Plays a Crucial Role at the Step of Membrane Tubulation during Cell Plate Formation
- PMID: 28584166
- PMCID: PMC5502459
- DOI: 10.1105/tpc.17.00108
SH3 Domain-Containing Protein 2 Plays a Crucial Role at the Step of Membrane Tubulation during Cell Plate Formation
Abstract
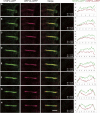
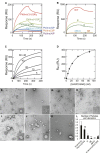
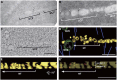
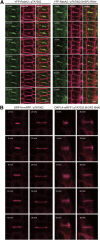
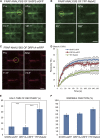
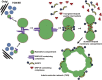
During cytokinesis in plants, trans-Golgi network-derived vesicles accumulate at the center of dividing cells and undergo various structural changes to give rise to the planar cell plate. However, how this conversion occurs at the molecular level remains elusive. In this study, we report that SH3 Domain-Containing Protein 2 (SH3P2) in Arabidopsis thaliana plays a crucial role in converting vesicles to the planar cell plate. SH3P2 RNAi plants showed cytokinesis-defective phenotypes and produced aggregations of vesicles at the leading edge of the cell plate. SH3P2 localized to the leading edge of the cell plate, particularly the constricted or curved regions of the cell plate. The BAR domain of SH3P2 induced tubulation of vesicles. SH3P2 formed a complex with dynamin-related protein 1A (DRP1A) and affected DRP1A accumulation to the cell plate. Based on these results, we propose that SH3P2 functions together with DRP1A to convert the fused vesicles to tubular structures during cytokinesis.
© 2017 American Society of Plant Biologists. All rights reserved.
Figures



References
-
- Abramoff M.D., Magelhaes P.J., Ram S.J. (2004). Image processing with ImageJ. Biophotonics International 11: 36–42.
-
- Aoyama T., Chua N.H. (1997). A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11: 605–612. - PubMed
-
- Bolte S., Talbot C., Boutte Y., Catrice O., Read N.D., Satiat-Jeunemaitre B. (2004). FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J. Microsc. 214: 159–173. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

