X-ray structures of fructosyl peptide oxidases revealing residues responsible for gating oxygen access in the oxidative half reaction
- PMID: 28584265
- PMCID: PMC5459902
- DOI: 10.1038/s41598-017-02657-5
X-ray structures of fructosyl peptide oxidases revealing residues responsible for gating oxygen access in the oxidative half reaction
Abstract
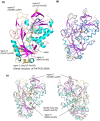
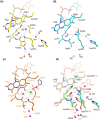
Current enzymatic systems for quantifying glycated hemoglobin are based on the FAD-containing enzyme fructosyl peptide oxidase (FPOX). FPOX has substrate specificity for fructosyl-α N-valyl-histidine derived from proteolytic digestion of the N-terminus of the HbA1c β-chain. This study reports the X-ray structures of the wild-type and Asn56Ala (N56A) mutant of Phaeosphaeria nodorum fructosyl peptide oxidase (PnFPOX) to elucidate the residues responsible for the oxidative half-reaction. N56A showed decreased oxidase activity compared to the wild -type, while its dye-mediated dehydrogenase activity was higher than that of wild type. In wild-type PnFPOX, Asn56 forms a hydrogen bond with Lys274, thereby preventing it from forming a salt bridge with Asp54. By contrast, Lys274 of PnFPOX N56A moves toward Asp54, and they approach each other to form a salt bridge at a distance of 2.92-3.35 Å. Site-directed mutagenesis studies and protein channel analysis suggest that Asp54 assists in accepting oxygen properly at the position of the bound water molecule in the main oxygen channel. These results reveal that Asn56 in PnFPOX is essential for maintaining an effective oxygen accession path, and support the role of Asp54 as a gate keeper that cooperates with Lys274 to enable oxygen to reach the active site properly.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Engineering fructosyl peptide oxidase to improve activity toward the fructosyl hexapeptide standard for HbA1c measurement.Mol Biotechnol. 2013 Jul;54(3):939-43. doi: 10.1007/s12033-012-9644-2. Mol Biotechnol. 2013. PMID: 23335126
-
Construction of engineered fructosyl peptidyl oxidase for enzyme sensor applications under normal atmospheric conditions.Biotechnol Lett. 2012 Mar;34(3):491-7. doi: 10.1007/s10529-011-0787-1. Epub 2011 Nov 4. Biotechnol Lett. 2012. PMID: 22052257
-
Engineering an efficient mutant of Eupenicillium terrenum fructosyl peptide oxidase for the specific determination of hemoglobin A1c.Appl Microbiol Biotechnol. 2019 Feb;103(4):1725-1735. doi: 10.1007/s00253-018-9529-9. Epub 2019 Jan 3. Appl Microbiol Biotechnol. 2019. PMID: 30607487
-
A comprehensive review on fructosyl peptide oxidase as an important enzyme for present hemoglobin A1c assays.Biotechnol Appl Biochem. 2025 Feb;72(1):268-281. doi: 10.1002/bab.2647. Epub 2024 Aug 4. Biotechnol Appl Biochem. 2025. PMID: 39099239 Review.
-
Amine oxidation by d-arginine dehydrogenase in Pseudomonas aeruginosa.Arch Biochem Biophys. 2017 Oct 15;632:192-201. doi: 10.1016/j.abb.2017.06.013. Epub 2017 Jun 15. Arch Biochem Biophys. 2017. PMID: 28625766 Review.
Cited by
-
Creation of haemoglobin A1c direct oxidase from fructosyl peptide oxidase by combined structure-based site specific mutagenesis and random mutagenesis.Sci Rep. 2019 Jan 30;9(1):942. doi: 10.1038/s41598-018-37806-x. Sci Rep. 2019. PMID: 30700768 Free PMC article.
-
Alteration of Electron Acceptor Preferences in the Oxidative Half-Reaction of Flavin-Dependent Oxidases and Dehydrogenases.Int J Mol Sci. 2020 May 27;21(11):3797. doi: 10.3390/ijms21113797. Int J Mol Sci. 2020. PMID: 32471202 Free PMC article. Review.
-
In Silico Engineering of Enzyme Access Tunnels.Methods Mol Biol. 2022;2397:203-225. doi: 10.1007/978-1-0716-1826-4_11. Methods Mol Biol. 2022. PMID: 34813066
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

