Sulfate radicals enable a non-enzymatic Krebs cycle precursor
- PMID: 28584880
- PMCID: PMC5455955
- DOI: 10.1038/s41559-017-0083
Sulfate radicals enable a non-enzymatic Krebs cycle precursor
Abstract
The evolutionary origins of the tricarboxylic acid cycle (TCA), or Krebs cycle, are so far unclear. Despite a few years ago, the existence of a simple non-enzymatic Krebs-cycle catalyst has been dismissed 'as an appeal to magic', citrate and other intermediates have meanwhile been discovered on a carbonaceous meteorite and do interconvert non-enzymatically. To identify the non-enzymatic Krebs cycle catalyst, we used combinatorial, quantitative high-throughput metabolomics to systematically screen iron and sulfate reaction milieus that orient on Archean sediment constituents. TCA cycle intermediates are found stable in water and in the presence of most iron and sulfate species, including simple iron-sulfate minerals. However, we report that TCA intermediates undergo 24 interconversion reactions in the presence of sulfate radicals that form from peroxydisulfate. The non-enzymatic reactions critically cover a topology as present in the Krebs cycle, the glyoxylate shunt and the succinic semialdehyde pathways. Assembled in a chemical network, the reactions achieve more than ninety percent carbon recovery. Our results show that a non-enzymatic precursor for the Krebs cycle is biologically sensible, efficient, and forms spontaneously in the presence of sulfate radicals.
Conflict of interest statement
Competing financial interests The authors declare no competing financial interest.
Figures
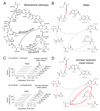

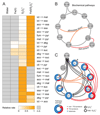

Comment in
-
Do sulfate radicals really enable a non-enzymatic Krebs cycle precursor?Nat Ecol Evol. 2019 Feb;3(2):138. doi: 10.1038/s41559-018-0791-0. Nat Ecol Evol. 2019. PMID: 30697001 No abstract available.
References
-
- Krebs HA, Johnson WA. The role of citric acid in intermediate metabolism in animal tissues. FEBS Lett. 1980;117(Suppl):K1–10. - PubMed
-
- Huynen MA, Dandekar T, Bork P. Variation and evolution of the citric-acid cycle: a genomic perspective. Trends Microbiol. 1999;7:281–291. - PubMed
-
- Meléndez-Hevia E, Waddell TG, Cascante M. The puzzle of the Krebs citric acid cycle: assembling the pieces of chemically feasible reactions, and opportunism in the design of metabolic pathways during evolution. J Mol Evol. 1996;43:293–303. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

