Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes
- PMID: 28585113
- PMCID: PMC5799125
- DOI: 10.1007/s12264-017-0145-y
Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes
Abstract
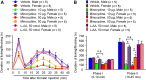
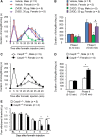
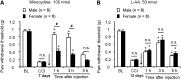
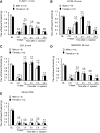
Increasing evidence suggests that spinal microglia regulate pathological pain in males. In this study, we investigated the effects of several microglial and astroglial modulators on inflammatory and neuropathic pain following intrathecal injection in male and female mice. These modulators were the microglial inhibitors minocycline and ZVEID (a caspase-6 inhibitor) and the astroglial inhibitors L-α-aminoadipate (L-AA, an astroglial toxin) and carbenoxolone (a connexin 43 inhibitor), as well as U0126 (an ERK kinase inhibitor) and D-JNKI-1 (a c-Jun N-terminal kinase inhibitor). We found that spinal administration of minocycline or ZVEID, or Caspase6 deletion, reduced formalin-induced inflammatory and nerve injury-induced neuropathic pain primarily in male mice. In contrast, intrathecal L-AA reduced neuropathic pain but not inflammatory pain in both sexes. Intrathecal U0126 and D-JNKI-1 reduced neuropathic pain in both sexes. Nerve injury caused spinal upregulation of the astroglial markers GFAP and Connexin 43 in both sexes. Collectively, our data confirmed male-dominant microglial signaling but also revealed sex-independent astroglial signaling in the spinal cord in inflammatory and neuropathic pain.
Keywords: Astrocytes; Microglia; Sex difference; Spinal cord.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

