Deletion of ghrelin prevents aging-associated obesity and muscle dysfunction without affecting longevity
- PMID: 28585250
- PMCID: PMC5506439
- DOI: 10.1111/acel.12618
Deletion of ghrelin prevents aging-associated obesity and muscle dysfunction without affecting longevity
Abstract
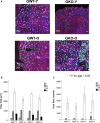
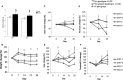
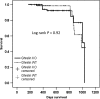
During aging, decreases in energy expenditure and locomotor activity lead to body weight and fat gain. Aging is also associated with decreases in muscle strength and endurance leading to functional decline. Here, we show that lifelong deletion of ghrelin prevents development of obesity associated with aging by modulating food intake and energy expenditure. Ghrelin deletion also attenuated the decrease in phosphorylated adenosine monophosphate-activated protein kinase (pAMPK) and downstream mediators in muscle, and increased the number of type IIa (fatigue resistant, oxidative) muscle fibers, preventing the decline in muscle strength and endurance seen with aging. Longevity was not affected by ghrelin deletion. Treatment of old mice with pharmacologic doses of ghrelin increased food intake, body weight, and muscle strength in both ghrelin wild-type and knockout mice. These findings highlight the relevance of ghrelin during aging and identify a novel AMPK-dependent mechanism for ghrelin action in muscle.
Keywords: Sarcopenia; frailty; growth hormone; growth hormone secretagogue receptor; inflammation; wasting.
© 2017 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures



References
-
- Ballak SB, Degens H, de Haan A, Jaspers RT (2014) Aging related changes in determinants of muscle force generating capacity: a comparison of muscle aging in men and male rodents. Ageing Res. Rev. 14, 43–55. - PubMed
-
- Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G (2005) Ghrelin regulates mitochondrial‐lipid metabolism gene expression and tissue fat distribution favoring triglyceride deposition in liver but not skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 288, E228–235. - PubMed
-
- Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE (2004) Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes. Res. 12, 1995–2004. - PubMed
-
- Beharka AA, Meydani M, Wu D, Leka LS, Meydani A, Meydani SN (2001) Interleukin‐6 production does not increase with age. J. Gerontol. A Biol. Sci. Med. Sci. 56, B81–B88. - PubMed
-
- Bergmeister KD, Groger M, Aman M, Willensdorfer A, Manzano‐Szalai K, Salminger S, Aszmann OC (2016) Automated muscle fiber type population analysis with ImageJ of whole rat muscles using rapid myosin heavy chain immunohistochemistry. Muscle Nerve 54, 292–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

