Direct-acting antivirals for chronic hepatitis C
- PMID: 28585310
- PMCID: PMC6484383
- DOI: 10.1002/14651858.CD012143.pub2
Direct-acting antivirals for chronic hepatitis C
Update in
-
Direct-acting antivirals for chronic hepatitis C.Cochrane Database Syst Rev. 2017 Sep 18;9(9):CD012143. doi: 10.1002/14651858.CD012143.pub3. Cochrane Database Syst Rev. 2017. PMID: 28922704 Free PMC article.
Abstract
Background: Millions of people worldwide suffer from hepatitis C, which can lead to severe liver disease, liver cancer, and death. Direct-acting antivirals (DAAs) are relatively new and expensive interventions for chronic hepatitis C, and preliminary results suggest that DAAs may eradicate hepatitis C virus (HCV) from the blood (sustained virological response). However, it is still questionable if eradication of hepatitis C virus in the blood eliminates hepatitis C in the body, and improves survival and leads to fewer complications.
Objectives: To assess the benefits and harms of DAAs in people with chronic HCV.
Search methods: We searched for all published and unpublished trials in The Cochrane Hepato-Biliary Group Controlled Trials Register, CENTRAL, MEDLINE, Embase, Science Citation Index Expanded, LILACS, and BIOSIS; the Chinese Biomedical Literature Database (CBM), China Network Knowledge Information (CNKI), the Chinese Science Journal Database (VIP), Google Scholar, The Turning Research into Practice (TRIP) Database, ClinicalTrials.gov, European Medicines Agency (EMA) (www.ema.europa.eu/ema/), WHO International Clinical Trials Registry Platform (www.who.int/ictrp), the Food and Drug Administration (FDA) (www.fda.gov), and pharmaceutical company sources for ongoing or unpublished trials. Searches were last run in October 2016.
Selection criteria: Randomised clinical trials comparing DAAs versus no intervention or placebo, alone or with co-interventions, in adults with chronic HCV. We included trials irrespective of publication type, publication status, and language.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. Our primary outcomes were hepatitis C-related morbidity, serious adverse events, and quality of life. Our secondary outcomes were all-cause mortality, ascites, variceal bleeding, hepato-renal syndrome, hepatic encephalopathy, hepatocellular carcinoma, non-serious adverse events (each reported separately), and sustained virological response. We systematically assessed risks of bias, performed Trial Sequential Analysis, and followed an eight-step procedure to assess thresholds for statistical and clinical significance. The overall quality of the evidence was evaluated using GRADE.
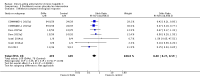
Main results: We included a total of 138 trials randomising a total of 25,232 participants. The 138 trials assessed the effects of 51 different DAAs. Of these, 128 trials employed matching placebo in the control group. All included trials were at high risk of bias. Eighty-four trials involved DAAs on the market or under development (13,466 participants). Fifty-seven trials administered withdrawn or discontinued DAAs. Trial participants were treatment-naive (95 trials), treatment-experienced (17 trials), or both treatment-naive and treatment-experienced (24 trials). The HCV genotypes were genotype 1 (119 trials), genotype 2 (eight trials), genotype 3 (six trials), genotype 4 (nine trials), and genotype 6 (one trial). We identified two ongoing trials.Meta-analysis of the effects of all DAAs on the market or under development showed no evidence of a difference when assessing hepatitis C-related morbidity or all-cause mortality (OR 3.72, 95% CI 0.53 to 26.18, P = 0.19, I² = 0%, 2,996 participants, 11 trials, very low-quality evidence). As there were no data on hepatitis C-related morbidity and very few data on mortality (DAA 15/2377 (0.63%) versus control 1/617 (0.16%)), it was not possible to perform Trial Sequential Analysis on hepatitis C-related morbidity or all-cause mortality.Meta-analysis of all DAAs on the market or under development showed no evidence of a difference when assessing serious adverse events (OR 0.93, 95% CI 0.75 to 1.15, P = 0.52, I² = 0%, 15,817 participants, 43 trials, very low-quality evidence). The Trial Sequential Analysis showed that the cumulative Z-score crossed the trial sequential boundary for futility, showing that there was sufficient information to rule out that DAAs compared with placebo reduced the relative risk of a serious adverse event by 20%. The only DAA that showed a significant difference on risk of serious adverse events when meta-analysed separately was simeprevir (OR 0.62, 95% CI 0.45 to 0.86). However, Trial Sequential Analysis showed that there was not enough information to confirm or reject a relative risk reduction of 20%, and when one trial with an extreme result was excluded, then the meta-analysis result showed no evidence of a difference.DAAs on the market or under development seemed to reduce the risk of no sustained virological response (RR 0.44, 95% CI 0.37 to 0.52, P < 0.00001, I² = 77%, 6886 participants, 32 trials, very low-quality evidence) and Trial Sequential Analysis confirmed this meta-analysis result.Only 1/84 trials on the market or under development assessed the effects of DAAs on health-related quality of life (SF-36 mental score and SF-36 physical score).Withdrawn or discontinued DAAs had no evidence of a difference when assessing hepatitis C-related morbidity and all-cause mortality (OR 0.64, 95% CI 0.23 to 1.79, P = 0.40, I² = 0%; 5 trials, very low-quality evidence). However, withdrawn DAAs seemed to increase the risk of serious adverse events (OR 1.45, 95% CI 1.22 to 1.73, P = 0.001, I² = 0%, 29 trials, very low-quality evidence), and Trial Sequential Analysis confirmed this meta-analysis result.Most of all outcome results were short-term results; therefore, we could neither confirm nor reject any long-term effects of DAAs. None of the 138 trials provided useful data to assess the effects of DAAs on the remaining secondary outcomes (ascites, variceal bleeding, hepato-renal syndrome, hepatic encephalopathy, and hepatocellular carcinoma).
Authors' conclusions: Overall, DAAs on the market or under development do not seem to have any effects on risk of serious adverse events. Simeprevir may have beneficial effects on risk of serious adverse event. In all remaining analyses, we could neither confirm nor reject that DAAs had any clinical effects. DAAs seemed to reduce the risk of no sustained virological response. The clinical relevance of the effects of DAAs on no sustained virological response is questionable, as it is a non-validated surrogate outcome. All trials and outcome results were at high risk of bias, so our results presumably overestimate benefit and underestimate harm. The quality of the evidence was very low.
Conflict of interest statement
JCJ: none declared. EN: none declared. JF: none declared. KK: none declared. KF: none declared. GH: none declared. GP: none declared. SD: none declared. KW: none declared. MB: none declared. GB: none declared. SK: none declared. JP: none declared. DN: none declared. RK: none declared. CG: none declared.
Figures



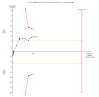


































































































Comment in
-
Response to the Cochrane systematic review on DAA-based treatment of chronic hepatitis C.J Hepatol. 2017 Oct;67(4):663-664. doi: 10.1016/j.jhep.2017.06.022. Epub 2017 Jun 29. J Hepatol. 2017. PMID: 28669753 No abstract available.
-
New hepatitis C antiviral treatments eliminate the virus.Lancet. 2017 Jul 22;390(10092):358-359. doi: 10.1016/S0140-6736(17)31817-2. Epub 2017 Jul 7. Lancet. 2017. PMID: 28689663 No abstract available.
-
What is the impact of treatment for hepatitis C virus infection?Lancet. 2017 Jul 8;390(10090):107-109. doi: 10.1016/S0140-6736(17)31762-2. Lancet. 2017. PMID: 28699578 No abstract available.
-
IDSA/AASLD Response to Cochrane Review on Direct-Acting Antivirals for Hepatitis C.Clin Infect Dis. 2017 Nov 13;65(11):1773-1775. doi: 10.1093/cid/cix620. Clin Infect Dis. 2017. PMID: 29020156 No abstract available.
References
References to studies included in this review
-
- Brogan AJ, Talbird SE, Thompson JR, Miller JD, Rubin J, Deniz B. Cost‐effectiveness of telaprevir combination therapy for chronic hepatitis C. PLoS ONE 2014;9(3):e90295. - PMC - PubMed
- Curtis S, Cure S, Gavart S, Dearden L, Fleischmann J, Ouwens M, et al. The cost‐effectiveness of telaprevir (TVR) in combination with pegylated interferon‐alfa and ribavirin (PR) for the treatment of genotype 1 chronic hepatitis C patients. Journal of Hepatology2012; Vol. 56, issue Suppl 2:S434.
- Bisceglie AM, Dusheiko GM, Muir AJ, Zeuzem S, Marcellin P, Bzowej NH. Telaprevir in combination with peginterferon alfa‐2a and ribavirin: analyses of pre‐defined subpopulations in the phase 3 ADVANCE trial. Gastroenterology 2011;140(5 Suppl 1):S908.
- Dusheiko GM, Bengtsson L, Adda N, Kauffman R, Jacobson IM. Telaprevir in combination with peginterferon and ribavirin in genotype 1 HCV treatment‐naive patients: final results of phase 3 Advance study. Gut 2011;60(Suppl 2):A32‐3.
- Everson G, Dusheiko G, Pol S, Nelson D, Muir A, Andreone P, et al. Telaprevir in combination with peginterferon alfa‐2a and ribavirin increased sustained virologic response in genotype 1 chronic HCV and allowed for shorter treatment duration in treatment‐naive and prior relapser patients. American Journal of Gastroenterology 2011;106(2S):S113‐4.
- Jacobson IM, McHutchison JG, Dusheiko G, Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. New England Journal of Medicine 2011;364(25):2405‐16. - PubMed
- Jacobson IM, McHutchison JG, Dusheiko GM, Bisceglie AM, Reddy R, Bzowej NH, et al. Telaprevir in combination with peginterferon and ribavirin in genotype 1 HCV treatment‐naive patients: final results of phase 3 ADVANCE study. Hepatology 2010;52(Suppl S1):427A.
- Kieffer TL, Bartels DJ, Sullivan J, Adiwijaya BS, Zhang EZ, Tigges A, et al. Clinical virology results from telaprevir phase 3 study ADVANCE. Hepatology 2010;52(Suppl S1):879A.
- Luo D, Zeuzem S, Jacobson IM, Sherman K, Adda N, Wright CI, et al. High concordance between SVR12 and SVR24 in patients receiving telaprevir plus peginterferon and ribavirin in three phase iii clinical trials: Advance, Illuminate and Realize. Journal of Hepatology 2012;56(Suppl 2):S446‐7.
- Marcellin P, Jacobson IM, Zeuzem S, Bzowej N, Muir AJ, Dusheiko G. Telaprevir in combination with peginterferon alfa‐2a and ribavirin: analyses of pre‐defined subpopulations in the phase 3 ADVANCE trial. Journal of Hepatology 2011;54(Suppl 1):S183‐4.
- Rizzetto M, Andreone P, Colombo M, Scuteri A, Ciancio A, Smedile A, et al. ADVANCE study: final results of the phase III trial with telaprevir in combination with PEG‐IFN and RBV in treatment‐naive genotype 1 HCV. Digestive and Liver Disease 2011;43(Suppl 2):S69.
- Sulkowski MS, Reddy R, Afdhal NH, Bisceglie AM, Zeuzem S, Poordad F, et al. Anemia had no effect on efficacy outcomes in treatment‐naive patients who received telaprevir‐based regimen in the Advance and Illuminate phase 3 studies. Journal of Hepatology 2011;54(Suppl 1):S195.
-
- Brogan AJ, Talbird SE, Thompson JR, Miller JD, Rubin J, Deniz B. Cost‐effectiveness of telaprevir combination therapy for chronic hepatitis C. PLoS ONE 2014;9(3):e90295. - PMC - PubMed
- Curtis S, Cure S, Gavart S, Dearden L, Fleischmann J, Ouwens M, et al. The cost‐effectiveness of telaprevir (TVR) in combination with pegylated interferon‐alfa and ribavirin (PR) for the treatment of genotype 1 chronic hepatitis C patients. Journal of Hepatology2012; Vol. 56, issue Suppl 2:S434.
- Bisceglie AM, Dusheiko GM, Muir AJ, Zeuzem S, Marcellin P, Bzowej NH. Telaprevir in combination with peginterferon alfa‐2a and ribavirin: analyses of pre‐defined subpopulations in the phase 3 ADVANCE trial. Gastroenterology 2011;140(5 Suppl 1):S908.
- Dusheiko GM, Bengtsson L, Adda N, Kauffman R, Jacobson IM. Telaprevir in combination with peginterferon and ribavirin in genotype 1 HCV treatment‐naive patients: final results of phase 3 Advance study. Gut 2011;60(Suppl 2):A32‐3.
- Everson G, Dusheiko G, Pol S, Nelson D, Muir A, Andreone P, et al. Telaprevir in combination with peginterferon alfa‐2a and ribavirin increased sustained virologic response in genotype 1 chronic HCV and allowed for shorter treatment duration in treatment‐naive and prior relapser patients. American Journal of Gastroenterology 2011;106(2S):S113‐4.
- Jacobson IM, McHutchison JG, Dusheiko G, Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. New England Journal of Medicine 2011;364(25):2405‐16. - PubMed
- Jacobson IM, McHutchison JG, Dusheiko GM, Bisceglie AM, Reddy R, Bzowej NH, et al. Telaprevir in combination with peginterferon and ribavirin in genotype 1 HCV treatment‐naive patients: final results of phase 3 ADVANCE study. Hepatology 2010;52(Suppl S1):427A.
- Kieffer TL, Bartels DJ, Sullivan J, Adiwijaya BS, Zhang EZ, Tigges A, et al. Clinical virology results from telaprevir phase 3 study ADVANCE. Hepatology 2010;52(Suppl S1):879A.
- Luo D, Zeuzem S, Jacobson IM, Sherman K, Adda N, Wright CI, et al. High concordance between SVR12 and SVR24 in patients receiving telaprevir plus peginterferon and ribavirin in three phase iii clinical trials: Advance, Illuminate and Realize. Journal of Hepatology 2012;56(Suppl 2):S446‐7.
- Marcellin P, Jacobson IM, Zeuzem S, Bzowej N, Muir AJ, Dusheiko G. Telaprevir in combination with peginterferon alfa‐2a and ribavirin: analyses of pre‐defined subpopulations in the phase 3 ADVANCE trial. Journal of Hepatology 2011;54(Suppl 1):S183‐4.
- Rizzetto M, Andreone P, Colombo M, Scuteri A, Ciancio A, Smedile A, et al. ADVANCE study: final results of the phase III trial with telaprevir in combination with PEG‐IFN and RBV in treatment‐naive genotype 1 HCV. Digestive and Liver Disease 2011;43(Suppl 2):S69.
- Sulkowski MS, Reddy R, Afdhal NH, Bisceglie AM, Zeuzem S, Poordad F, et al. Anemia had no effect on efficacy outcomes in treatment‐naive patients who received telaprevir‐based regimen in the Advance and Illuminate phase 3 studies. Journal of Hepatology 2011;54(Suppl 1):S195.
-
- Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient‐reported outcomes (HCV‐PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of Life Research 2014;23(3):877‐86. - PubMed
- Gaultier I, Cohen DE, Bhathena A, Idler K, Larsen LM, Podsadecki T, et al. The effect of IL28B polymorphisms on virologic response to treatment with pegylated interferon alpha‐2A and ribavirin (SOC) added to ABT‐450/ritonavir (ABT‐450/R), ABT‐333, or ABT‐072. Journal of Hepatology 2011;54(Suppl 1):S523.
- Pilot‐Matias TJ, Tripathi RL, Dekhtyar T, Menon RM, Gaultier IA, Cohen DE, et al. Genotypic and phenotypic characterization of NS3 variants selected in HCV‐infected patients treated with ABT‐450. Journal of Hepatology 2011;54(Suppl 1):S485‐6.
-
- Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient‐reported outcomes (HCV‐PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of Life Research 2014;23(3):877‐86. - PubMed
- Gaultier I, Cohen DE, Bhathena A, Idler K, Larsen LM, Podsadecki T, et al. The effect of IL28B polymorphisms on virologic response to treatment with pegylated interferon alpha‐2A and ribavirin (SOC) added to ABT‐450/ritonavir (ABT‐450/R), ABT‐333, or ABT‐072. Journal of Hepatology 2011;54(Suppl 1):S523.
- Pilot‐Matias TJ, Tripathi RL, Dekhtyar T, Menon RM, Gaultier IA, Cohen DE, et al. Genotypic and phenotypic characterization of NS3 variants selected in HCV‐infected patients treated with ABT‐450. Journal of Hepatology 2011;54(Suppl 1):S485‐6.
-
- Anderson RT, Baran RW, Erickson P, Revicki DA, Dietz B, Gooch K. Psychometric evaluation of the hepatitis C virus patient‐reported outcomes (HCV‐PRO) instrument: validity, responsiveness, and identification of the minimally important difference in a phase 2 clinical trial. Quality of Life Research 2014;23(3):877‐86. - PubMed
- Gaultier I, Cohen DE, Bhathena A, Idler K, Larsen LM, Podsadecki T, et al. The effect of IL28B polymorphisms on virologic response to treatment with pegylated interferon alpha‐2A and ribavirin (SOC) added to ABT‐450/ritonavir (ABT‐450/R), ABT‐333, or ABT‐072. Journal of Hepatology 2011;54(Suppl 1):S523.
- Pilot‐Matias TJ, Tripathi RL, Dekhtyar T, Menon RM, Gaultier IA, Cohen DE, et al. Genotypic and phenotypic characterization of NS3 variants selected in HCV‐infected patients treated with ABT‐450. Journal of Hepatology 2011;54(Suppl 1):S485‐6.
References to studies excluded from this review
-
- Asselah T, Hassanein TI, Qaqish RB, Feld JJ, Hezode C, Zeuzem S, et al. Efficacy and safety of ombitasvir/paritaprevir/ritonavir co‐administered with ribavirin in adults with genotype 4 chronic hepatitis C infection and cirrhosis (AGATE‐I). Hepatology 2015;62(Suppl S1):563A‐4A. - PubMed
-
- Wyles D, Ruane P, Sulkowski M, Dieterich D, Luetkemeyer A, Morgan T, et al. Daclatasvir in combination with sofosbuvir for HIV/HCV coinfection: ALLY‐2 study. Topics in Antiviral Medicine2015; Vol. 23, issue e‐1:62.
-
- Jensen DM, Brunda M, Elston R, Gane EJ, George J, Glavini K, et al. Interferon‐free regimen containing setrobuvir (STV) in combination with ritonavir‐boosted danoprevir (DNVr) and ribavirin (R) with or without mericitabine (MCB) in HCV genotype (G)1 treatment‐naive patients: interim SVR4 results from the ANNAPURNA study. Hepatology 2013;58(S1):741A.
-
- APRICOT Study Group. APRICOT Study evaluated. Surprisingly high virologic response in HIV‐HCV coinfection. MMW Fortschritte der Medizin 2004;146(Spec No 1):74‐5. - PubMed
-
- Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, Demicco M, et al. Sofosbuvir with pegylated interferon alfa‐2a and ribavirin for treatment‐naive patients with hepatitis C genotype‐1 infection (ATOMIC): an open‐label, randomised, multicentre phase 2 trial. Lancet 2013;381(9883):2100‐7. - PubMed
References to ongoing studies
-
- Izumi N, LaTaillade M, Chayama K, Toyota J, Mochida S, Kawada N, et al. First report of peginterferon lambda/ribavirin in combination with either daclatasvir or asunaprevir in HCV genotype 1 Japanese subjects: early sustained virologic response (SVR4) results from the D‐LITE Japanese sub‐study. Hepatology 2012;56(S1):310A.
-
- Lawitz E, Rodriguez‐Torres M, Nguyen T, Sheikh A, Tobias H, Galati J, et al. A phase II study of samatasvir (IDX719) in combination with simeprevir and ribavirin in treatment‐naive HCV‐infected subjects with genotypes 1B and 4 (helix‐1 study). Journal of Hepatology 2014;60(1 Suppl S):S495‐6.
Additional references
-
- Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. New England Journal of Medicine 2014;370(20):1889‐98. - PubMed
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

