Liver-Directed Human Amniotic Epithelial Cell Transplantation Improves Systemic Disease Phenotype in Hurler Syndrome Mouse Model
- PMID: 28585336
- PMCID: PMC5689764
- DOI: 10.1002/sctm.16-0449
Liver-Directed Human Amniotic Epithelial Cell Transplantation Improves Systemic Disease Phenotype in Hurler Syndrome Mouse Model
Abstract
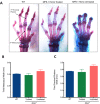
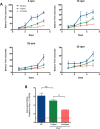
Mucopolysaccharidosis type 1 (MPS1) is an inherited lysosomal storage disorder caused by a deficiency in the glycosaminoglycan (GAG)-degrading enzyme α-l-iduronidase (IDUA). In affected patients, the systemic accumulation of GAGs results in skeletal dysplasia, neurological degeneration, multiple organ dysfunction, and early death. Current therapies, including enzyme replacement and bone marrow transplant, improve life expectancy but the benefits to skeletal and neurological phenotypes are limited. In this study, we tested the therapeutic efficacy of liver-directed transplantation of a placental stem cell, which possesses multilineage differentiation potential, low immunogenicity, and high lysosomal enzyme activity. Unfractionated human amniotic epithelial cells (hAECs) were transplanted directly into the liver of immunodeficient Idua knockout mouse neonates. The hAECs engraftment was immunohistochemically confirmed with anti-human mitochondria staining. Enzyme activity assays indicated that hAECs transplantation restored IDUA function in the liver and significantly decreased urinary GAG excretion. Histochemical and micro-computed tomography analyses revealed reduced GAG deposition in the phalanges joints and composition/morphology improvement of cranial and facial bones. Neurological assessment in the hAEC treated mice showed significant improvement of sensorimotor coordination in the hAEC treated mice compared to untreated mice. Results confirm that partial liver cell replacement with placental stem cells can provide long-term (>20 weeks) and systemic restoration of enzyme function, and lead to significant phenotypic improvement in the MPS1 mouse model. This preclinical data indicate that liver-directed placental stem cell transplantation may improve skeletal and neurological phenotypes of MPS1 patients. Stem Cells Translational Medicine 2017;6:1583-1594.
Keywords: Amnion; Cell transplantation; Congenital; Metabolism; Mucopolysaccharidosis I; Placenta.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures



References
-
- Meikle PJ, Hopwood JJ, Clague AE et al. Prevalence of lysosomal storage disorders. JAMA 1999;281:249–254. - PubMed
-
- Wraith JE, Jones S. Mucopolysaccharidosis type I. Pediatr Endocrinol Rev 2014;12(suppl 1):102–106. - PubMed
-
- Pastores GM. Laronidase (Aldurazyme): Enzyme replacement therapy for mucopolysaccharidosis type I. Expert Opin Biol Ther 2008;8:1003–1009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

