Punicalagin protects bovine endometrial epithelial cells against lipopolysaccharide-induced inflammatory injury
- PMID: 28585424
- PMCID: PMC5482043
- DOI: 10.1631/jzus.B1600224
Punicalagin protects bovine endometrial epithelial cells against lipopolysaccharide-induced inflammatory injury
Abstract
Objective: Bovine endometritis is one of the most common reproductive disorders in cattle. The aim of this study was to investigate the anti-inflammation potential of punicalagin in lipopolysaccharide (LPS)-induced bovine endometrial epithelial cells (bEECs) and to uncover the underlying mechanisms.
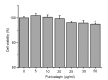
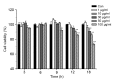
Methods: bEECs were stimulated with different concentrations (1, 10, 30, 50, and 100 μg/ml) of LPS for 3, 6, 9, 12, and 18 h. MTT assay was used to assess cell viability and to identify the conditions for inflammatory injury and effective concentrations of punicalagin. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to assess gene expression of pro-inflammatory cytokines. Western blotting was used to assess levels of inflammation-related proteins.
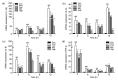
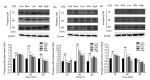
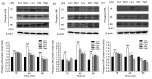
Results: Treatment of bEECs with 30 µg/ml LPS for 12 h induced cell injury and reduced cell viability. Punicalagin (5, 10, or 20 µg/ml) pretreatment significantly decreased LPS-induced productions of interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α (TNF-α) in bEECs. Molecular research showed that punicalagin inhibited the activation of the upstream mediator nuclear factor-κB (NF-κB) by suppressing the production of inhibitor κBα (IκBα) and phosphorylation of p65. Results also indicated that punicalagin can suppress the phosphorylation of mitogen-activated protein kinases (MAPKs) including p38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK).
Conclusions: Punicalagin may attenuate LPS-induced inflammatory injury and provide a potential option for the treatment of dairy cows with Escherichia coli endometritis.
Keywords: Bovine endometrial epithelial cell; Cytokine; Inflammatory injury; Punicalagin.
Conflict of interest statement
All institutional and national guidelines for the care and use of laboratory animals were followed.
Figures

Similar articles
-
Sodium houttuyfonate inhibits inflammation by blocking the MAPKs/NF-κB signaling pathways in bovine endometrial epithelial cells.Res Vet Sci. 2015 Jun;100:245-51. doi: 10.1016/j.rvsc.2015.04.004. Epub 2015 Apr 21. Res Vet Sci. 2015. PMID: 25935757
-
Cortisol inhibits NF-κB and MAPK pathways in LPS activated bovine endometrial epithelial cells.Int Immunopharmacol. 2018 Mar;56:71-77. doi: 10.1016/j.intimp.2018.01.021. Epub 2018 Jan 23. Int Immunopharmacol. 2018. PMID: 29367089
-
IFN-τ Attenuates LPS-Induced Endometritis by Restraining HMGB1/NF-κB Activation in bEECs.Inflammation. 2021 Aug;44(4):1478-1489. doi: 10.1007/s10753-021-01433-y. Epub 2021 Feb 18. Inflammation. 2021. PMID: 33604776
-
Docosahexaenoic acid attenuates LPS-stimulated inflammatory response by regulating the PPARγ/NF-κB pathways in primary bovine mammary epithelial cells.Res Vet Sci. 2017 Jun;112:7-12. doi: 10.1016/j.rvsc.2016.12.011. Epub 2017 Jan 5. Res Vet Sci. 2017. PMID: 28095338 Review.
-
Symposium review: Mechanisms linking metabolic stress with innate immunity in the endometrium.J Dairy Sci. 2018 Apr;101(4):3655-3664. doi: 10.3168/jds.2017-13135. Epub 2017 Sep 6. J Dairy Sci. 2018. PMID: 28888597 Review.
Cited by
-
Ureaplasma urealyticum-derived lipid-associated membrane proteins introduce IL-6, IL-8, and TNF-α cytokines into human amniotic epithelial cells via Toll-like receptor 2.J Zhejiang Univ Sci B. 2018 Aug.;19(8):654-661. doi: 10.1631/jzus.B1800005. J Zhejiang Univ Sci B. 2018. PMID: 30070088 Free PMC article.
-
Punicalagin relieves hepatic injury by antioxidation and enhancement of autophagy in diet-induced nonalcoholic steatohepatitis.Sci Rep. 2025 Apr 25;15(1):14516. doi: 10.1038/s41598-025-98044-6. Sci Rep. 2025. PMID: 40280981 Free PMC article.
-
Different effects of cortisol on pro-inflammatory gene expressions in LPS-, heat-killed E.coli-, or live E.coli-stimulated bovine endometrial epithelial cells.BMC Vet Res. 2020 Jan 9;16(1):9. doi: 10.1186/s12917-020-2231-z. BMC Vet Res. 2020. PMID: 31918707 Free PMC article.
-
Structural uterine changes in postpartum endometritis in cows.Vet World. 2018 Nov;11(10):1473-1478. doi: 10.14202/vetworld.2018.1473-1478. Epub 2018 Oct 22. Vet World. 2018. PMID: 30532504 Free PMC article.
-
Punicalagin Exerts Protective Effects against Ankylosing Spondylitis by Regulating NF-κB-TH17/JAK2/STAT3 Signaling and Oxidative Stress.Biomed Res Int. 2020 Sep 23;2020:4918239. doi: 10.1155/2020/4918239. eCollection 2020. Biomed Res Int. 2020. PMID: 33029510 Free PMC article.
References
-
- Brodzki P, Bochniarz M, Brodzki A, et al. Trueperella pyogenes and Escherichia coli as an etiological factor of endometritis in cows and the susceptibility of these bacteria to selected antibiotics. Pol J Vet Sci. 2014;17(4):657–664. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

