Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents
- PMID: 28585535
- PMCID: PMC5467173
- DOI: 10.1038/ncomms15623
Blood-brain-barrier spheroids as an in vitro screening platform for brain-penetrating agents
Abstract
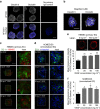
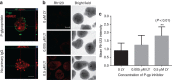
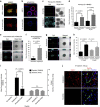
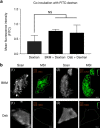
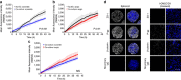
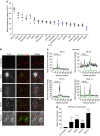
Culture-based blood-brain barrier (BBB) models are crucial tools to enable rapid screening of brain-penetrating drugs. However, reproducibility of in vitro barrier properties and permeability remain as major challenges. Here, we report that self-assembling multicellular BBB spheroids display reproducible BBB features and functions. The spheroid core is comprised mainly of astrocytes, while brain endothelial cells and pericytes encase the surface, acting as a barrier that regulates transport of molecules. The spheroid surface exhibits high expression of tight junction proteins, VEGF-dependent permeability, efflux pump activity and receptor-mediated transcytosis of angiopep-2. In contrast, the transwell co-culture system displays comparatively low levels of BBB regulatory proteins, and is unable to discriminate between the transport of angiopep-2 and a control peptide. Finally, we have utilized the BBB spheroids to screen and identify BBB-penetrant cell-penetrating peptides (CPPs). This robust in vitro BBB model could serve as a valuable next-generation platform for expediting the development of CNS therapeutics.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Abbott N. J., Rönnbäck L. & Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006). - PubMed
-
- Wolburg H. & Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul. Pharmacol. 38, 323–337 (2002). - PubMed
-
- Schinkel A. H. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 36, 179–194 (1999). - PubMed
-
- Begley D. J. & Brightman M. W. Structural and functional aspects of the blood-brain barrier. Prog. Drug Res. 61, 39–78 (2003). - PubMed
-
- Zlokovic B. V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

