Therapeutic Potential of Matrix Metalloproteinase Inhibition in Breast Cancer
- PMID: 28585723
- PMCID: PMC5621753
- DOI: 10.1002/jcb.26185
Therapeutic Potential of Matrix Metalloproteinase Inhibition in Breast Cancer
Abstract
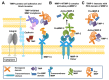
Matrix metalloproteinases (MMPs) are a family of zinc endopeptidases that cleave nearly all components of the extracellular matrix as well as many other soluble and cell-associated proteins. MMPs have been implicated in normal physiological processes, including development, and in the acquisition and progression of the malignant phenotype. Disappointing results from a series of clinical trials testing small molecule, broad spectrum MMP inhibitors as cancer therapeutics led to a re-evaluation of how MMPs function in the tumor microenvironment, and ongoing research continues to reveal that these proteins play complex roles in cancer development and progression. It is now clear that effective targeting of MMPs for therapeutic benefit will require selective inhibition of specific MMPs. Here, we provide an overview of the MMP family and its biological regulators, the tissue inhibitors of metalloproteinases (TIMPs). We then summarize recent research from model systems that elucidate how specific MMPs drive the malignant phenotype of breast cancer cells, including acquisition of cancer stem cell features and induction of the epithelial-mesenchymal transition, and we also outline clinical studies that implicate specific MMPs in breast cancer outcomes. We conclude by discussing ongoing strategies for development of inhibitors with therapeutic potential that are capable of selectively targeting the MMPs most responsible for tumor promotion, with special consideration of the potential of biologics including antibodies and engineered proteins based on the TIMP scaffold. J. Cell. Biochem. 118: 3531-3548, 2017. © 2017 The Authors. Journal of Cellular Biochemistry Published by Wiley Periodicals, Inc.
Keywords: BREAST CANCER; CANCER BIOMARKERS; EPITHELIAL MESENCHYMAL TRANSITION; MATRIX METALLOPROTEINASES; MMP INHIBITORS; TISSUE INHIBITORS OF METALLOPROTEINASES; TUMOR MICROENVIRONMENT; TUMOR PROGRESSION.
© 2017 The Authors. Journal of Cellular Biochemistry Published by Wiley Periodicals, Inc.
Figures



References
-
- Alvarez OA, Carmichael DF, DeClerck YA. 1990. Inhibition of collagenolytic activity and metastasis of tumor cells by a recombinant human tissue inhibitor of metalloproteinases. J Natl Cancer Inst 82(7):589–595. - PubMed
-
- Annes JP, Munger JS, Rifkin DB. 2003. Making sense of latent TGFbeta activation. J Cell Sci 116(Pt 2):217–224. - PubMed
-
- Amour A, Knight CG, English WR, Webster A, Slocombe PM, Knauper V, Docherty AJ, Becherer JD, Blobel CP, Murphy G. 2002. The enzymatic activity of ADAM8 and ADAM9 is not regulated by TIMPs. FEBS Lett 524(1‐3):154–158. - PubMed
-
- Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. 2009. Neutrophil MMP‐9 proenzyme, unencumbered by TIMP‐1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF‐2)/FGFR‐2 pathway. J Biol Chem 284(38):25854–25866. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

