Evaluation of the Potential for Drug Interactions With Patiromer in Healthy Volunteers
- PMID: 28585859
- PMCID: PMC5555446
- DOI: 10.1177/1074248417691135
Evaluation of the Potential for Drug Interactions With Patiromer in Healthy Volunteers
Abstract
Introduction: Patiromer is a potassium-binding polymer that is not systemically absorbed; however, it may bind coadministered oral drugs in the gastrointestinal tract, potentially reducing their absorption.
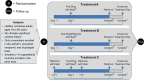
Methods: Twelve randomized, open-label, 3-period, 3-sequence crossover studies were conducted in healthy volunteers to evaluate the effect of patiromer (perpetrator drug) on absorption and single-dose pharmacokinetics (PK) of drugs (victims) that might be commonly used with patiromer. Subjects received victim drug alone, victim drug administered together with patiromer 25.2 g (highest approved dose), and victim drug administered 3 hours before patiromer 25.2 g. The primary PK endpoints were area under the curve (AUC), extrapolated to infinity (AUC0-∞), and maximum concentration ( Cmax). Results were reported as 90% confidence intervals (CIs) about the geometric mean AUC0-∞ and Cmax ratios with prespecified equivalence limits of 80% to 125%.
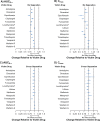
Results: Overall, 370 subjects were enrolled, with 365 receiving ≥1 dose of patiromer; 351 subjects completed the studies and all required treatments. When coadministered with patiromer, the 90% CIs for AUC0-∞ remained within 80% to 125% for 9 drugs (amlodipine, cinacalcet, clopidogrel, furosemide, lithium, metoprolol, trimethoprim, verapamil, and warfarin). The AUC0-∞ point estimate ratios for levothyroxine and metformin with patiromer coadministration were ≥80%, with the lower bounds of the 90% CIs at 76.8% and 72.8%, respectively. For ciprofloxacin, the point estimate for AUC0-∞ was 71.5% (90% CI: 65.3-78.4). For 8 of 12 drugs, point estimates for Cmax were ≥80% with patiromer coadministration; for ciprofloxacin, clopidogrel, metformin, and metoprolol, the point estimates were <80%. When patiromer was administered 3 hours after each victim drug, the 90% CIs for AUC0-∞ and Cmax for each drug were within the prespecified 80% to 125% limits.
Conclusion: For 9 of the 12 drugs coadministered with patiromer, there were no clinically significant drug-drug interactions. For 3 drugs (ciprofloxacin, levothyroxine, and metformin), a 3-hour separation between patiromer and their administration resulted in no clinically significant drug-drug interactions.
Keywords: absorption; dose separation; drug–drug interactions; hyperkalemia; patiromer; potassium-binder.
Conflict of interest statement
Figures


References
-
- Loutradis C, Tolika P, Skodra A, Avdelidou A, Sarafidis PA. Prevalence of hyperkalemia in diabetic and non-diabetic patients with chronic kidney disease: a nested case-control study. Am J Nephrol. 2015;42(5):351–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

