Cellular uptake of nanoparticles: journey inside the cell
- PMID: 28585944
- PMCID: PMC5593313
- DOI: 10.1039/c6cs00636a
Cellular uptake of nanoparticles: journey inside the cell
Abstract
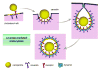
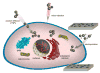
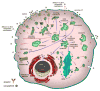
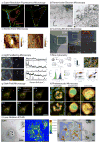
Nanoscale materials are increasingly found in consumer goods, electronics, and pharmaceuticals. While these particles interact with the body in myriad ways, their beneficial and/or deleterious effects ultimately arise from interactions at the cellular and subcellular level. Nanoparticles (NPs) can modulate cell fate, induce or prevent mutations, initiate cell-cell communication, and modulate cell structure in a manner dictated largely by phenomena at the nano-bio interface. Recent advances in chemical synthesis have yielded new nanoscale materials with precisely defined biochemical features, and emerging analytical techniques have shed light on nuanced and context-dependent nano-bio interactions within cells. In this review, we provide an objective and comprehensive account of our current understanding of the cellular uptake of NPs and the underlying parameters controlling the nano-cellular interactions, along with the available analytical techniques to follow and track these processes.
Conflict of interest statement
O.C.F. declares financial interests in Selecta Biosciences, Tarveda Therapeutics and Placon Therapeutics.
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

