Long-term tremor therapy for Parkinson and essential tremor with sensor-guided botulinum toxin type A injections
- PMID: 28586370
- PMCID: PMC5460844
- DOI: 10.1371/journal.pone.0178670
Long-term tremor therapy for Parkinson and essential tremor with sensor-guided botulinum toxin type A injections
Abstract
Objective: Current pharmacological agents used to treat Parkinson disease (PD) tremor and essential tremor (ET) provide suboptimal benefit and are commonly associated with significant adverse effects. Botulinum toxin type A (BoNT-A) has been shown to be effective for wrist tremor though functionally bothersome muscle weakness frequently occurs. This is the longest study to date demonstrating that BoNT-A therapy coupled with kinematic guidance can provide efficacious outcomes for upper limb tremor with minimized unwanted weakness.
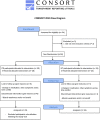
Methods: A total of 28 PD and 24 ET participants with bothersome, disabling tremor, received six serial BoNT-A treatments every 16 weeks starting at week 0 with a follow-up visit 6 weeks following a treatment, totaling 96 weeks. Clinical scales, including Fahn-Tolosa-Marin tremor rating scale (FTM), and sensor-based tremor assessments were conducted at each visit. Kinematics was utilized to identify which arm muscles contributed to the tremulous movements and the experienced injector used clinical expertise in determining BoNT-A dosages.
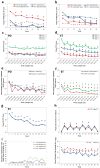
Results: Following BoNT-A treatment, clinical ratings of tremor severity and functional ability (FTM) showed significant improvements following the first treatment which was maintained up to week 96 in PD and ET. Kinematics detected a significant reduction in PD and ET tremor amplitudes by 70% and 76% over the treatment course, respectively. By objectively distinguishing tremulous muscles and tremor severity, adverse effects were limited to mild perceived weakness by participants in injected muscles during follow-ups. Following the fourth treatment, BoNT-A dosages in flexor and extensor wrist muscles and biceps were reduced for those experiencing residual weakness which ultimately did not interfere with tremor relief or arm function.
Conclusions: Kinematics is an objective method that can aid clinicians in assessing and determining optimal BoNT-A parameters to alleviate both PD and ET tremor. BoNT-A injections are tolerable and effective when focal therapy regimens are determined and optimized kinematically over a long-term.
Conflict of interest statement
Figures
Similar articles
-
Botulinum Toxin Type A Injections as Monotherapy for Upper Limb Essential Tremor Using Kinematics.Can J Neurol Sci. 2018 Jan;45(1):11-22. doi: 10.1017/cjn.2017.260. Epub 2017 Nov 21. Can J Neurol Sci. 2018. PMID: 29157315 Clinical Trial.
-
Functional Ability Improved in Essential Tremor by IncobotulinumtoxinA Injections Using Kinematically Determined Biomechanical Patterns - A New Future.PLoS One. 2016 Apr 21;11(4):e0153739. doi: 10.1371/journal.pone.0153739. eCollection 2016. PLoS One. 2016. PMID: 27101283 Free PMC article. Clinical Trial.
-
Developing a Consistent, Reproducible Botulinum Toxin Type A Dosing Method for Upper Limb Tremor by Kinematic Analysis.Toxins (Basel). 2021 Apr 8;13(4):264. doi: 10.3390/toxins13040264. Toxins (Basel). 2021. PMID: 33917695 Free PMC article.
-
Novel Botulinum Toxin Injection Protocols for Parkinson Tremor and Essential Tremor - the Yale Technique and Sensor-Based Kinematics Procedure for Safe and Effective Treatment.Tremor Other Hyperkinet Mov (N Y). 2020 Dec 31;10:61. doi: 10.5334/tohm.582. Tremor Other Hyperkinet Mov (N Y). 2020. PMID: 33442486 Free PMC article. Review.
-
Use of Botulinum Toxin in Upper-Limb Tremor: Systematic Review and Perspectives.Toxins (Basel). 2024 Sep 13;16(9):392. doi: 10.3390/toxins16090392. Toxins (Basel). 2024. PMID: 39330850 Free PMC article.
Cited by
-
Tremor in Parkinson's Disease: From Pathophysiology to Advanced Therapies.Tremor Other Hyperkinet Mov (N Y). 2022 Sep 13;12:29. doi: 10.5334/tohm.712. eCollection 2022. Tremor Other Hyperkinet Mov (N Y). 2022. PMID: 36211804 Free PMC article. Review.
-
Transitioning from Unilateral to Bilateral Upper Limb Tremor Therapy for Parkinson's Disease and Essential Tremor Using Botulinum Toxin: Case Series.Toxins (Basel). 2018 Sep 27;10(10):394. doi: 10.3390/toxins10100394. Toxins (Basel). 2018. PMID: 30262746 Free PMC article. Clinical Trial.
-
Clinical assessment of upper limb impairments and functional capacity in Parkinson's disease: a systematic review.Arq Neuropsiquiatr. 2023 Nov;81(11):1008-1015. doi: 10.1055/s-0043-1772769. Epub 2023 Oct 29. Arq Neuropsiquiatr. 2023. PMID: 37899049 Free PMC article.
-
Current and Future Neuropharmacological Options for the Treatment of Essential Tremor.Curr Neuropharmacol. 2020;18(6):518-537. doi: 10.2174/1570159X18666200124145743. Curr Neuropharmacol. 2020. PMID: 31976837 Free PMC article. Review.
-
Botulinum Toxin for the Treatment of Hand Tremor.Toxins (Basel). 2018 Jul 19;10(7):299. doi: 10.3390/toxins10070299. Toxins (Basel). 2018. PMID: 30029483 Free PMC article.
References
-
- Montgomery EB. Deep brain stimulation programming: principles and practice. 1st ed USA: Oxford University Press; 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

