Tomato ethylene sensitivity determines interaction with plant growth-promoting bacteria
- PMID: 28586422
- PMCID: PMC5737082
- DOI: 10.1093/aob/mcx052
Tomato ethylene sensitivity determines interaction with plant growth-promoting bacteria
Abstract
Background and aims: Plant growth-promoting bacteria (PGPB) are soil micro-organisms able to interact with plants and stimulate their growth, positively affecting plant physiology and development. Although ethylene plays a key role in plant growth, little is known about the involvement of ethylene sensitivity in bacterial inoculation effects on plant physiology. Thus, the present study was pursued to establish whether ethylene perception is critical for plant-bacteria interaction and growth induction by two different PGPB strains, and to assess the physiological effects of these strains in juvenile and mature tomato ( Solanum lycopersicum ) plants.
Methods: An experiment was performed with the ethylene-insensitive tomato never ripe and its isogenic wild-type line in which these two strains were inoculated with either Bacillus megaterium or Enterobacter sp. C7. Plants were grown until juvenile and mature stages, when biomass, stomatal conductance, photosynthesis as well as nutritional, hormonal and metabolic statuses were analysed.
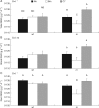
Key results: Bacillus megaterium promoted growth only in mature wild type plants. However, Enterobacter C7 PGPB activity affected both wild-type and never ripe plants. Furthermore, PGPB inoculation affected physiological parameters and root metabolite levels in juvenile plants; meanwhile plant nutrition was highly dependent on ethylene sensitivity and was altered at the mature stage. Bacillus megaterium inoculation improved carbon assimilation in wild-type plants. However, insensitivity to ethylene compromised B. megaterium PGPB activity, affecting photosynthetic efficiency, plant nutrition and the root sugar content. Nevertheless, Enterobacter C7 inoculation modified the root amino acid content in addition to stomatal conductance and plant nutrition.
Conclusions: Insensitivity to ethylene severely impaired B. megaterium interaction with tomato plants, resulting in physiological modifications and loss of PGPB activity. In contrast, Enterobacter C7 inoculation stimulated growth independently of ethylene perception and improved nitrogen assimilation in ethylene-insensitive plants. Thus, ethylene sensitivity is a determinant for B. megaterium , but is not involved in Enterobacter C7 PGPB activity.
Keywords: Bacillus megaterium; Enterobacter; Solanum lycorpersicum (tomato); ethylene; plant growth-promoting bacteria (PGPB); plant nutrition.
© The Author 2017. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For Permissions, please email: journals.permissions@oup.com
Figures



References
-
- Adesemoye AO, Kloepper JW.. 2009. Plant–microbes interactions in enhanced fertilizer-use efficiency. Applied Microbiology and Biotechnology 85: 1–12. - PubMed
-
- Armada E, Portela G, Roldán A, Azcón R.. 2014a. Combined use of beneficial soil microorganism and agrowaste residue to cope with plant water limitation under semiarid conditions. Geoderma 232–234: 640–648.
-
- Armada E, Roldán A, Azcon R.. 2014b. Differential activity of autochthonous bacteria in controlling drought stress in native Lavandula and Salvia plants species under drought conditions in natural arid soil. Microbial Ecology 67: 410–420. - PubMed
-
- Aroca R, Ruiz-Lozano JM.. 2009. Induction of plant tolerance to semi-arid environments by beneficial soil microorganisms. A review (E) In: Lichtfouse E, ed. Climate change, intercropping, pest control and beneficial microorganisms. Berlin: Springer, 121–135.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

