Endothelial-to-Osteoblast Conversion Generates Osteoblastic Metastasis of Prostate Cancer
- PMID: 28586644
- PMCID: PMC5512590
- DOI: 10.1016/j.devcel.2017.05.005
Endothelial-to-Osteoblast Conversion Generates Osteoblastic Metastasis of Prostate Cancer
Abstract
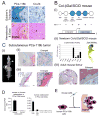
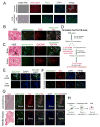
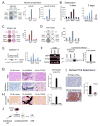
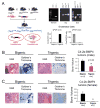
Prostate cancer (PCa) bone metastasis is frequently associated with bone-forming lesions, but the source of the osteoblastic lesions remains unclear. We show that the tumor-induced bone derives partly from tumor-associated endothelial cells that have undergone endothelial-to-osteoblast (EC-to-OSB) conversion. The tumor-associated osteoblasts in PCa bone metastasis specimens and patient-derived xenografts (PDXs) were found to co-express endothelial marker Tie-2. BMP4, identified in PDX-conditioned medium, promoted EC-to-OSB conversion of 2H11 endothelial cells. BMP4 overexpression in non-osteogenic C4-2b PCa cells led to ectopic bone formation under subcutaneous implantation. Tumor-induced bone was reduced in trigenic mice (Tie2cre/Osxf/f/SCID) with endothelial-specific deletion of osteoblast cell-fate determinant OSX compared with bigenic mice (Osxf/f/SCID). Thus, tumor-induced EC-to-OSB conversion is one mechanism that leads to osteoblastic bone metastasis of PCa.
Keywords: bone metastasis; endothelial-to-osteoblast conversion; osteoblast; paracrine factors; prostate cancer; proteomics.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
C. Logothetis reports receiving a commercial research grant from Astellas, BMS, Sanofi, Janssen, Bayer, and Medivation; has received speakers bureau honoraria from Astellas, Janssen, Sanofi, and Bayer; and is a consultant/advisory board member for Astellas, Janssen, Sanofi, and Bayer. No potential conflicts of interest were disclosed by the other authors.
Figures


Comment in
-
The Bony Side of Endothelial Cells in Prostate Cancer.Dev Cell. 2017 Jun 5;41(5):451-452. doi: 10.1016/j.devcel.2017.05.015. Dev Cell. 2017. PMID: 28586639
References
-
- Aubin JE. Advances in the osteoblast lineage. Biochem Cell Biol. 1998;76:899–910. - PubMed
-
- Bourne S, Polak JM, Hughes SP, Buttery LD. Osteogenic differentiation of mouse embryonic stem cells: differential gene expression analysis by cDNA microarray and purification of osteoblasts by cadherin-11 magnetically activated cell sorting. Tissue Eng. 2004;10:796–806. - PubMed
-
- Buttery LD, Bourne S, Xynos JD, Wood H, Hughes FJ, Hughes SP, Episkopou V, Polak JM. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7:89–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

