The Effect of Positive SWOG Treatment Trials on Survival of Patients With Cancer in the US Population
- PMID: 28586789
- PMCID: PMC5710507
- DOI: 10.1001/jamaoncol.2017.0762
The Effect of Positive SWOG Treatment Trials on Survival of Patients With Cancer in the US Population
Abstract
Importance: Recently, tremendous prominence has been given to the investigation of the effect of different research processes as part of the Cancer Moonshot. More than half a century ago, the National Cancer Institute (NCI) established a network of publicly funded cancer cooperative research groups to systematically evaluate new treatments for efficacy and safety.
Objective: To examine the extent to which positive NCI-sponsored cancer treatment trials have benefited patients with cancer in the US population.
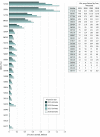
Design, setting, and participants: This investigation used study data from SWOG, an NCI-sponsored network cooperative research group. All treatment trials during SWOG's 60-year history (1956-2016) were identified for which the new, experimental therapy provided a statistically significant improvement in overall survival. It was assumed that the new, proven treatments from these trials established new standards for cancer care in the treatment community. Twenty-three positive SWOG treatment trials were identified from a variety of different disease settings.
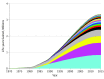
Main outcomes and measures: This study estimated population life-years gained from the 23 treatment trials through 2015 by mapping the effect of the new treatments onto the US cancer population using an area under the Kaplan-Meier survival curve approach that combined trial-specific hazard function and hazard ratio results, along with Surveillance, Epidemiology, and End Results program and life table data. Calculations were age adjusted. The US dollar return on investment was estimated as the ratio of the total investment by the NCI in the treatment trial program divided by the estimate of life-years gained.
Results: In total, 12 361 patients were enrolled to the 23 positive trials from 1965 to 2012. The study estimated that 3.34 million (95% confidence limit, 2.39-4.15 million) life-years were gained from these 23 trials through 2015. Estimates were greater than 2 million life-years gained under most model simulations. The US dollar return on investment was $125 per life-year gained.
Conclusions and relevance: SWOG treatment trials have had a substantial impact on population survival for patients with cancer over 60 years. The NCI's investment in its cancer cooperative group research program has provided exceptional value and benefit to the American public through its research programs generating positive cancer treatment trials.
Trial registration: clinicaltrials.gov Identifiers: NCT00004001, NCT00075764, and NCT00644228.
Conflict of interest statement
Figures
References
-
- National Cancer Institute Cancer trends progress report: person-years of life lost. http://www.progressreport.cancer.gov/end/life_lost. Accessed March 29, 2017.
-
- Nass SJ, Moses HL, Mendelsohn J, eds; Institute of Medicine Committee on Cancer Clinical Trials and the NCI Cooperative Group Program. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington, DC: National Academies Press; 2010. - PubMed
-
- SWOG Cancer Research Fast facts. http://www.swog.org/Media/SWOG-Fast-Facts.pdf. Published 2014. Accessed March 29, 2017.
-
- National Cancer Institute. Cancer Moonshot Blue Ribbon Panel Report http://www.cancer.gov/brp. Published 2016. Accessed March 29, 2017.
-
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. SEER data, 1973-2013. Released April 2016, based on the November 2015 submission. http://www.seer.cancer.gov. Accessed June 14, 2016.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical