Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment
- PMID: 28586821
- PMCID: PMC5817466
- DOI: 10.1001/jama.2017.7156
Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment
Abstract
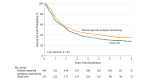
This study assesses overall survival associated with electronic patient-reported symptom monitoring vs usual care during routine cancer treatment.
Conflict of interest statement
Figures
Comment in
-
Patient-Reported Symptom Monitoring During Chemotherapy.JAMA. 2017 Nov 21;318(19):1935. doi: 10.1001/jama.2017.14899. JAMA. 2017. PMID: 29164248 No abstract available.
References
-
- Kotronoulas G, Kearney N, Maguire R, et al. . What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? a systematic review of controlled trials. J Clin Oncol. 2014;32(14):1480-1501. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

