Trigger-Responsive Gene Transporters for Anticancer Therapy
- PMID: 28587119
- PMCID: PMC5485767
- DOI: 10.3390/nano7060120
Trigger-Responsive Gene Transporters for Anticancer Therapy
Abstract
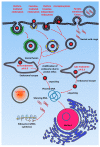
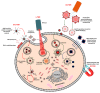
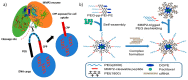
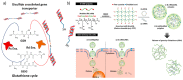
In the current era of gene delivery, trigger-responsive nanoparticles for the delivery of exogenous nucleic acids, such as plasmid DNA (pDNA), mRNA, siRNAs, and miRNAs, to cancer cells have attracted considerable interest. The cationic gene transporters commonly used are typically in the form of polyplexes, lipoplexes or mixtures of both, and their gene transfer efficiency in cancer cells depends on several factors, such as cell binding, intracellular trafficking, buffering capacity for endosomal escape, DNA unpacking, nuclear transportation, cell viability, and DNA protection against nucleases. Some of these factors influence other factors adversely, and therefore, it is of critical importance that these factors are balanced. Recently, with the advancements in contemporary tools and techniques, trigger-responsive nanoparticles with the potential to overcome their intrinsic drawbacks have been developed. This review summarizes the mechanisms and limitations of cationic gene transporters. In addition, it covers various triggers, such as light, enzymes, magnetic fields, and ultrasound (US), used to enhance the gene transfer efficiency of trigger-responsive gene transporters in cancer cells. Furthermore, the challenges associated with and future directions in developing trigger-responsive gene transporters for anticancer therapy are discussed briefly.
Keywords: anti-cancer; cationic polymer; gene delivery; glutathione; magnetic field; non-viral; photothermal; trigger-responsive; ultrasound.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
Similar articles
-
Intracellular Availability of pDNA and mRNA after Transfection: A Comparative Study among Polyplexes, Lipoplexes, and Lipopolyplexes.Mol Pharm. 2016 Sep 6;13(9):3153-63. doi: 10.1021/acs.molpharmaceut.6b00376. Epub 2016 Aug 22. Mol Pharm. 2016. PMID: 27486998
-
Polymers for nucleic acid transfer-an overview.Adv Genet. 2014;88:231-61. doi: 10.1016/B978-0-12-800148-6.00008-0. Adv Genet. 2014. PMID: 25409608 Review.
-
Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers.Front Pharmacol. 2018 Aug 21;9:971. doi: 10.3389/fphar.2018.00971. eCollection 2018. Front Pharmacol. 2018. PMID: 30186185 Free PMC article. Review.
-
Dual environment-responsive polyplex carriers for enhanced intracellular delivery of plasmid DNA.Biomacromolecules. 2012 Nov 12;13(11):3641-9. doi: 10.1021/bm301095a. Epub 2012 Oct 9. Biomacromolecules. 2012. PMID: 22994314
-
Physicochemical properties of polymers: An important system to overcome the cell barriers in gene transfection.Biopolymers. 2015 Jul;103(7):363-75. doi: 10.1002/bip.22638. Biopolymers. 2015. PMID: 25761628
Cited by
-
Gene therapy with RALA/iNOS composite nanoparticles significantly enhances survival in a model of metastatic prostate cancer.Cancer Nanotechnol. 2018;9(1):5. doi: 10.1186/s12645-018-0040-x. Epub 2018 Jun 1. Cancer Nanotechnol. 2018. PMID: 29899810 Free PMC article.
-
Ruthenium-containing supramolecular nanoparticles based on bipyridine-modified cyclodextrin and adamantyl PEI with DNA condensation properties.Nanoscale Res Lett. 2018 Dec 19;13(1):408. doi: 10.1186/s11671-018-2820-y. Nanoscale Res Lett. 2018. PMID: 30569227 Free PMC article.
-
Polydopamine-based nanoparticles with excellent biocompatibility for photothermally enhanced gene delivery.RSC Adv. 2018 Oct 9;8(60):34596-34602. doi: 10.1039/c8ra06916f. eCollection 2018 Oct 4. RSC Adv. 2018. PMID: 35548626 Free PMC article.
-
Core-Shell Nanoparticles as an Efficient, Sustained, and Triggered Drug-Delivery System.ACS Omega. 2017 Oct 31;2(10):6455-6463. doi: 10.1021/acsomega.7b01016. Epub 2017 Oct 6. ACS Omega. 2017. PMID: 30023520 Free PMC article.
References
-
- Hou S., Ziebacz N., Wieczorek S.A., Kalwarczyk E., Sashuk V., Kalwarczyk T., Kaminski T.S., Holyst R. Formation and structure of pei/DNA complexes: Quantitative analysis. Soft Matter. 2011;7:6967–6972. doi: 10.1039/c1sm05449j. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

