Chemical Methods to Knock Down the Amyloid Proteins
- PMID: 28587164
- PMCID: PMC6152772
- DOI: 10.3390/molecules22060916
Chemical Methods to Knock Down the Amyloid Proteins
Abstract
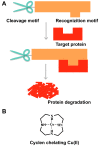
Amyloid proteins are closely related with amyloid diseases and do tremendous harm to human health. However, there is still a lack of effective strategies to treat these amyloid diseases, so it is important to develop novel methods. Accelerating the clearance of amyloid proteins is a favorable method for amyloid disease treatment. Recently, chemical methods for protein reduction have been developed and have attracted much attention. In this review, we focus on the latest progress of chemical methods that knock down amyloid proteins, including the proteolysis-targeting chimera (PROTAC) strategy, the "recognition-cleavage" strategy, the chaperone-mediated autophagy (CMA) strategy, the selectively light-activatable organic and inorganic molecules strategy and other chemical strategies.
Keywords: amyloid proteins; chemical methods; degradation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kisilevsky R. Biology of disease amyloidosis: A familiar problem in light of current pathogenic developments. Lab Investig. 1983;49:381–390. - PubMed
-
- Qi Q., Zhao T.X., An B.L., Liu X.Y., Zhong C. Self-assembly and morphological characterization of two-component functional amyloid proteins. Chin. Chem. Lett. 2016;12:008. doi: 10.1016/j.cclet.2016.12.008. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

