Flexible Proteins at the Origin of Life
- PMID: 28587235
- PMCID: PMC5492145
- DOI: 10.3390/life7020023
Flexible Proteins at the Origin of Life
Abstract
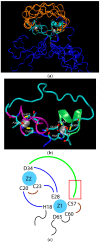
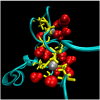
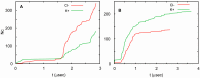
Almost all modern proteins possess well-defined, relatively rigid scaffolds that provide structural preorganization for desired functions. Such scaffolds require the sufficient length of a polypeptide chain and extensive evolutionary optimization. How ancestral proteins attained functionality, even though they were most likely markedly smaller than their contemporary descendants, remains a major, unresolved question in the origin of life. On the basis of evidence from experiments and computer simulations, we argue that at least some of the earliest water-soluble and membrane proteins were markedly more flexible than their modern counterparts. As an example, we consider a small, evolved in vitro ligase, based on a novel architecture that may be the archetype of primordial enzymes. The protein does not contain a hydrophobic core or conventional elements of the secondary structure characteristic of modern water-soluble proteins, but instead is built of a flexible, catalytic loop supported by a small hydrophilic core containing zinc atoms. It appears that disorder in the polypeptide chain imparts robustness to mutations in the protein core. Simple ion channels, likely the earliest membrane protein assemblies, could also be quite flexible, but still retain their functionality, again in contrast to their modern descendants. This is demonstrated in the example of antiamoebin, which can serve as a useful model of small peptides forming ancestral ion channels. Common features of the earliest, functional protein architectures discussed here include not only their flexibility, but also a low level of evolutionary optimization and heterogeneity in amino acid composition and, possibly, the type of peptide bonds in the protein backbone.
Keywords: ancestral enzyme; ancestral membrane protein; flexible protein; ion channels; primordial protein structure; protein ligase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Planning Implications Related to Sterilization-Sensitive Science Investigations Associated with Mars Sample Return (MSR).Astrobiology. 2022 Jun;22(S1):S112-S164. doi: 10.1089/AST.2021.0113. Epub 2022 May 19. Astrobiology. 2022. PMID: 34904892
-
Novel insights into the origin and diversification of photosynthesis based on analyses of conserved indels in the core reaction center proteins.Photosynth Res. 2017 Feb;131(2):159-171. doi: 10.1007/s11120-016-0307-1. Epub 2016 Sep 16. Photosynth Res. 2017. PMID: 27638319
-
Modular organization of α-toxins from scorpion venom mirrors domain structure of their targets, sodium channels.J Biol Chem. 2013 Jun 28;288(26):19014-27. doi: 10.1074/jbc.M112.431650. Epub 2013 May 1. J Biol Chem. 2013. PMID: 23637230 Free PMC article.
-
Molecular evolution before the origin of species.Prog Biophys Mol Biol. 2002 May-Jul;79(1-3):77-133. doi: 10.1016/s0079-6107(02)00012-3. Prog Biophys Mol Biol. 2002. PMID: 12225777 Review.
-
The origin and early evolution of membrane channels.Astrobiology. 2005 Feb;5(1):1-17. doi: 10.1089/ast.2005.5.1. Astrobiology. 2005. PMID: 15711166 Review.
Cited by
-
The Way forward for the Origin of Life: Prions and Prion-Like Molecules First Hypothesis.Life (Basel). 2021 Aug 25;11(9):872. doi: 10.3390/life11090872. Life (Basel). 2021. PMID: 34575021 Free PMC article.
-
The s-oph enzyme for efficient degradation of polyvinyl alcohol: soluble expression and catalytic properties.Mol Biol Rep. 2023 Oct;50(10):8523-8535. doi: 10.1007/s11033-023-08712-x. Epub 2023 Aug 29. Mol Biol Rep. 2023. PMID: 37644367
-
Physical Principles and Extant Biology Reveal Roles for RNA-Containing Membraneless Compartments in Origins of Life Chemistry.Biochemistry. 2018 May 1;57(17):2509-2519. doi: 10.1021/acs.biochem.8b00081. Epub 2018 Mar 21. Biochemistry. 2018. PMID: 29560725 Free PMC article.
-
The intramolecular hydrogen bonded-halogen bond: a new strategy for preorganization and enhanced binding.Chem Sci. 2018 Jun 21;9(26):5828-5836. doi: 10.1039/c8sc01973h. eCollection 2018 Jul 14. Chem Sci. 2018. PMID: 30079195 Free PMC article.
-
Intrinsically Disordered Proteins: Critical Components of the Wetware.Chem Rev. 2022 Mar 23;122(6):6614-6633. doi: 10.1021/acs.chemrev.1c00848. Epub 2022 Feb 16. Chem Rev. 2022. PMID: 35170314 Free PMC article. Review.
References
-
- Tiessen A., Pérez-Rodríguez P., Delaye-Arredondo L.J. Mathematical modeling and comparison of protein size distribution in different plant, animal, fungal and microbial species reveals a negative correlation between protein size and protein number, thus providing insight into the evolution of proteomes. BMC Res. Notes. 2012;5:85. doi: 10.1186/1756-0500-5-85. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

