Magnetic Lateral Flow Strip for the Detection of Cocaine in Urine by Naked Eyes and Smart Phone Camera
- PMID: 28587239
- PMCID: PMC5492392
- DOI: 10.3390/s17061286
Magnetic Lateral Flow Strip for the Detection of Cocaine in Urine by Naked Eyes and Smart Phone Camera
Abstract
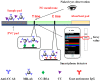
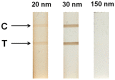
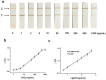
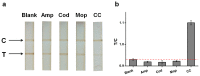
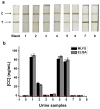
Magnetic lateral flow strip (MLFS) based on magnetic bead (MB) and smart phone camera has been developed for quantitative detection of cocaine (CC) in urine samples. CC and CC-bovine serum albumin (CC-BSA) could competitively react with MB-antibody (MB-Ab) of CC on the surface of test line of MLFS. The color of MB-Ab conjugate on the test line relates to the concentration of target in the competition immunoassay format, which can be used as a visual signal. Furthermore, the color density of the MB-Ab conjugate can be transferred into digital signal (gray value) by a smart phone, which can be used as a quantitative signal. The linear detection range for CC is 5-500 ng/mL and the relative standard deviations are under 10%. The visual limit of detection was 5 ng/mL and the whole analysis time was within 10 min. The MLFS has been successfully employed for the detection of CC in urine samples without sample pre-treatment and the result is also agreed to that of enzyme-linked immunosorbent assay (ELISA). With the popularization of smart phone cameras, the MLFS has large potential in the detection of drug residues in virtue of its stability, speediness, and low-cost.
Keywords: cocaine; magnetic bead; magnetic lateral flow strip; smart phone camera.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Floriani G., Gasparetto J.C., Pontarolo R., Goncalves A.G. Development and Validation of an HPLC-DAD Method for Simultaneous Determination of Cocaine, Benzoic acid, Benzoylecgonine and the Main Adulterants Found in Products based on Cocaine. Forensic. Sci. Int. 2014;235:32–39. doi: 10.1016/j.forsciint.2013.11.013. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

