Novel Structurally Related Flavones Augment Cell Death Induced by rhsTRAIL
- PMID: 28587286
- PMCID: PMC5486034
- DOI: 10.3390/ijms18061211
Novel Structurally Related Flavones Augment Cell Death Induced by rhsTRAIL
Abstract
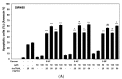
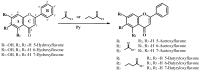
TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) was identified as a powerful activator of apoptosis in tumor cells and one of the most promising candidates for cancer therapy with no toxicity against normal tissues. However, many tumor cells are resistant to TRAIL-induced apoptosis. The aim of this work was to analyze the improvement of the anticancer effect of rhsTRAIL (recombinant human soluble TRAIL) by nine flavones: 5-Hydroxyflavone, 6-Hydroxyflavone, 7-Hydroxyflavone and their new synthetic derivatives 5-acetoxyflavone, 5-butyryloxyflavone, 6-acetoxyflavone, 6-butyryloxyflavone, 7-acetoxyflavone and 7-butyryloxyflavone. We examined the cytotoxic and apoptotic effects of rhsTRAIL enhanced by novel structurally-related flavones on SW480 and SW620 colon cancer cells using the3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test, the lactate dehydrogenase assay and annexin V-FITC fluorescence staining. We observed a slight difference in the activities of the flavones that was dependent on their chemical structure. Our study indicates that all nine flavones significantly augment cell death by rhsTRAIL (cytotoxicity range 36.8 ± 1.7%-91.4 ± 1.7%; apoptosis increase of 33.0 ± 0.7%-78.5 ± 0.9%). Our study demonstrates the potential use of tested flavones in TRAIL-based anticancer therapy and prevention.
Keywords: TRAIL; apoptosis; cancer cells; cytotoxicity; flavones.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

