Improvement of cytomegalovirus pp65 DNA vaccine efficacy by co-administration of siRNAs targeting BAK and BAX
- PMID: 28587400
- PMCID: PMC5450512
- DOI: 10.3892/etm.2017.4385
Improvement of cytomegalovirus pp65 DNA vaccine efficacy by co-administration of siRNAs targeting BAK and BAX
Abstract
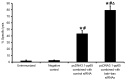
The efficacy of DNA vaccines may be improved by small interfering (si)RNA adjuvants targeting pro-apoptotic genes. The aim of the present study was to investigate the capacity of siRNAs targeting B-cell lymphoma 2 homologous antagonist killer (BAK) and B-cell lymphoma 2-associated X protein (BAX) to improve the efficacy of a cytomegalovirus (CMV) vaccine. BALB/c mice were divided into four groups (n=18 in each): unimmunized and immunized with pcDNA 3.1-pp65 expressing CMV 65 kDa matrix phosphoprotein and BAK + BAX siRNAs, pcDNA 3.1-pp65 and control siRNA, or control pcDNA 3.1 and BAK + BAX siRNAs. Immunizations were performed twice with an interval of 3 weeks. CMV-specific mouse splenocyte interferon (IFN)-γ secretion was assessed by ELISPOT; furthermore, an in vivo cytotoxic T lymphocyte assay was performed 2 weeks after the last immunization. After lethal CMV challenge of the mice, body weight, virus titers in the spleens and salivary glands as well as survival were recorded. The amount of splenocytes secreting IFN-γ in response to CMV pp65 peptides and specific lysis of peptide-pulsed target cells were significantly higher in mice administered pcDNA3.1-pp65 and BAK + BAX siRNAs than those in mice administered pcDNA3.1-pp65 and control siRNA (P<0.05 for each). After the virus challenge, the virus titers in the spleens and salivary glands of mice given pcDNA3.1-pp65 and BAK + BAX siRNAs were significantly lower than those in mice immunized with pcDNA3.1-pp65 and control siRNA (P<0.05 for each). Furthermore, mice immunized with pcDNA 3.1-pp65 and control siRNA or BAK + BAX siRNAs survived for longer, and at 21 days after lethal CMV challenge, 66 and 100% of these mice survived, respectively. These mice also experienced less weight loss compared with mice immunized with pcDNA3.1-pp65 and control siRNA (P<0.05). In conclusion, intradermal administration of siRNAs targeting BAK and BAX improved the efficacy of CMV pp65 DNA vaccine.
Keywords: BAK; BAX; DNA vaccine; cytomegalovirus; cytomegalovirus 65 kDa matrix phosphoprotein; small interfering RNA.
Figures


References
-
- Pereira L, Maidji E. Cytomegalovirus infection in the human placenta: Maternal immunity and developmentally regulated receptors on trophoblasts converge. Curr Top Microbiol Immunol. 2008;325:383–395. - PubMed
-
- Morello CS, Kelley LA, Munks MW, Hill AB, Spector DH. DNA immunization using highly conserved murine cytomegalovirus genes encoding homologs of human cytomegalovirus UL54 (DNA polymerase) and UL105 (helicase) elicits strong CD8 T-cell responses and is protective against systemic challenge. J Virol. 2007;81:7766–7775. doi: 10.1128/JVI.00633-07. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
