Hypertension with unsatisfactory sleep health (HUSH): study protocol for a randomized controlled trial
- PMID: 28587609
- PMCID: PMC5461741
- DOI: 10.1186/s13063-017-2001-9
Hypertension with unsatisfactory sleep health (HUSH): study protocol for a randomized controlled trial
Abstract
Background: Insomnia is common in primary care medical practices. Although behavioral treatments for insomnia are safe, efficacious, and recommended in practice guidelines, they are not widely-available, and their effects on comorbid medical conditions remain uncertain. We are conducting a pragmatic clinical trial to test the efficacy of two cognitive behavioral treatments for insomnia (Brief Behavioral Treatment for Insomnia (BBTI) and Sleep Healthy Using the Internet (SHUTi)) versus an enhanced usual care condition (EUC).
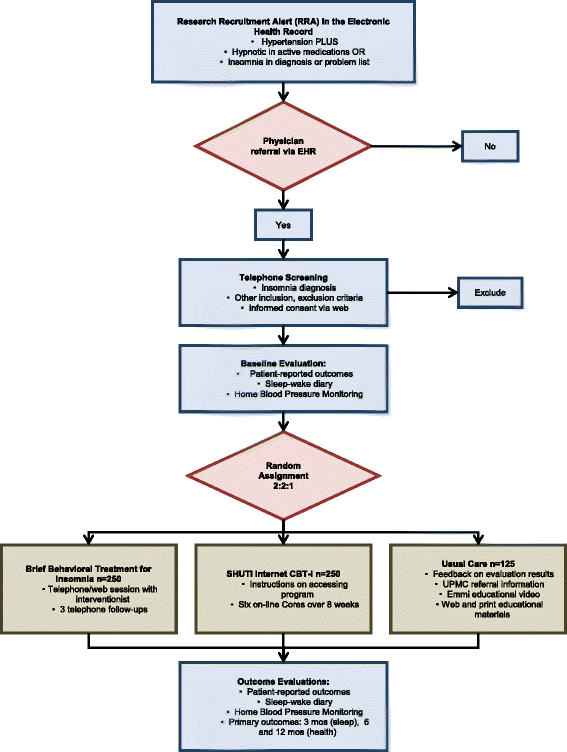
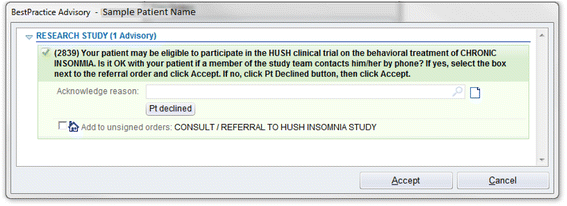
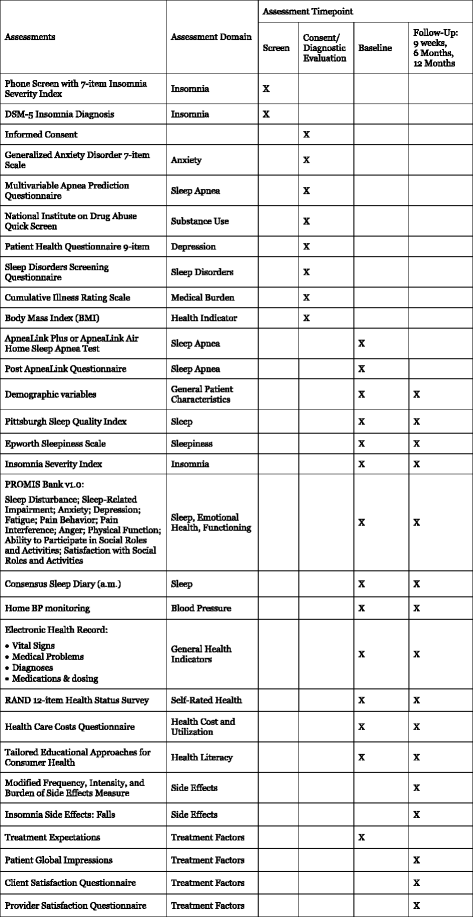
Methods/design: The study is a three-arm, parallel group, randomized controlled trial. Participants include 625 adults with hypertension and insomnia, recruited via electronic health records from primary care practices affiliated with a large academic medical center. After screening and baseline assessments, participants are randomized to treatment. BBTI is delivered individually with a live therapist via web-interface/telehealth sessions, while SHUTi is a self-guided, automated, interactive, web-based form of cognitive behavioral therapy for insomnia. Participants in EUC receive an individualized sleep report, educational resources, and an online educational video. Treatment outcomes are measured at 9 weeks, 6 months, and 12 months. The primary outcome is patient-reported sleep disturbances. Secondary outcomes include other self-reported sleep measures, home blood pressure, body mass index, quality of life, health functioning, healthcare utilization, and side effects.
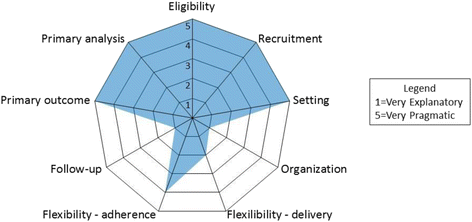
Discussion: This randomized clinical trial compares two efficacious insomnia interventions to EUC, and provides a cost-effective and efficient examination of their similarities and differences. The pragmatic orientation of this trial may impact sleep treatment delivery in real world clinical settings and advance the dissemination and implementation of behavioral sleep interventions.
Trial registration: ClinicalTrials.gov (Identifier: NCT02508129 ; Date Registered: July 21, 2015).
Keywords: Blood pressure; Hypertension; Sleep; Technology.
Figures
References
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical