Bactericidal effects of 310 nm ultraviolet light-emitting diode irradiation on oral bacteria
- PMID: 28587675
- PMCID: PMC5461700
- DOI: 10.1186/s12903-017-0382-5
Bactericidal effects of 310 nm ultraviolet light-emitting diode irradiation on oral bacteria
Abstract
Background: Ultraviolet (UV) light is used for phototherapy in dermatology, and UVB light (around 310 nm) is effective for treatment of psoriasis and atopic dermatitis. In addition, it is known that UVC light (around 265 nm) has a bactericidal effect, but little is known about the bactericidal effect of UVB light. In this study, we examined the bactericidal effects of UVB-light emitting diode (LED) irradiation on oral bacteria to explore the possibility of using a 310 nm UVB-LED irradiation device for treatment of oral infectious diseases.
Methods: We prepared a UVB (310 nm) LED device for intraoral use to examine bactericidal effects on Streptococcus mutans, Streptococcus sauguinis, Porphyromonas gingivalis, and Fusobacterium nucleatum and also to examine the cytotoxicity to a human oral epithelial cell line (Ca9-22). We also examined the production of nitric oxide and hydrogen peroxide from Ca9-22 cells after irradiation with UVB-LED light.
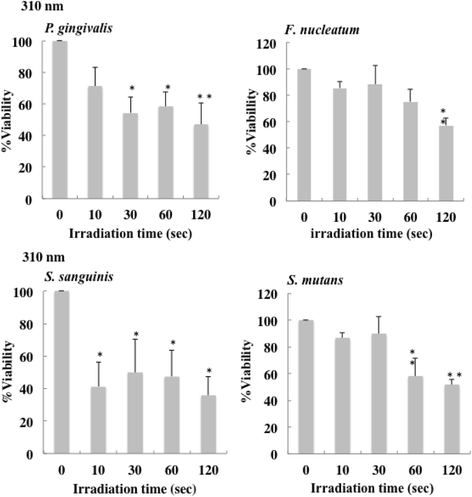
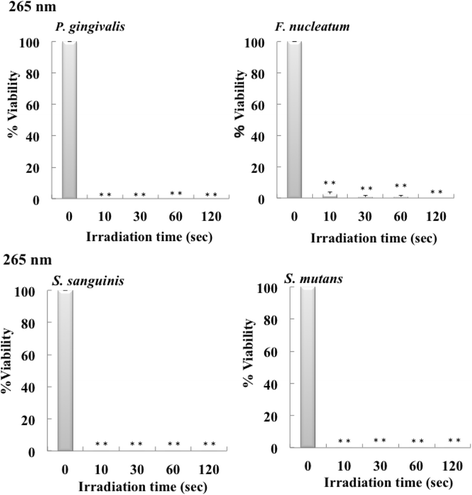
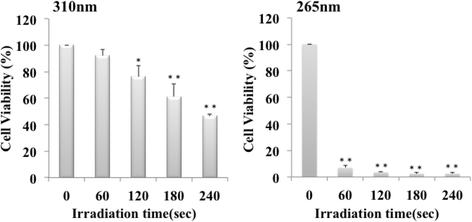
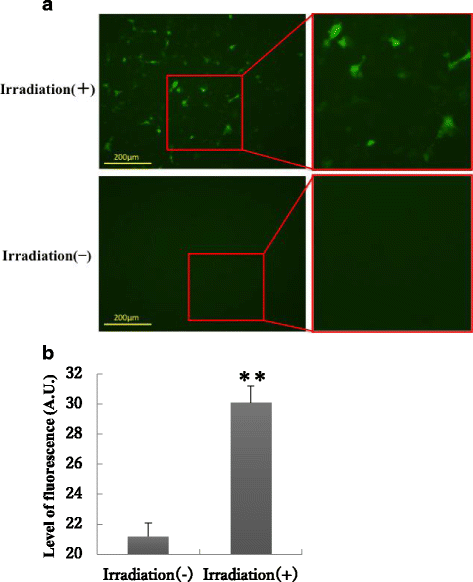
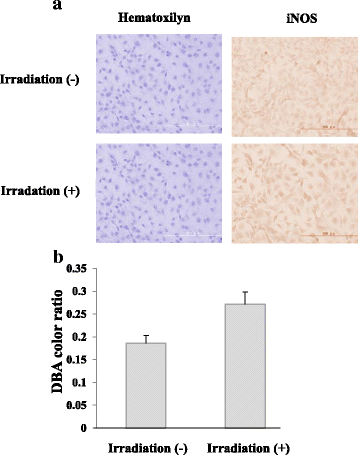
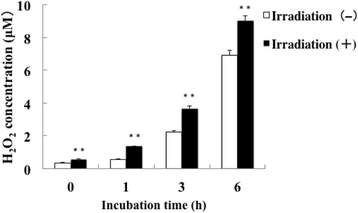
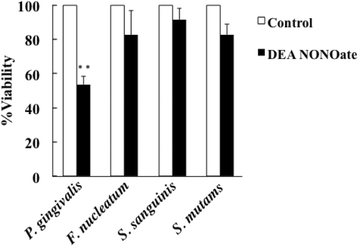
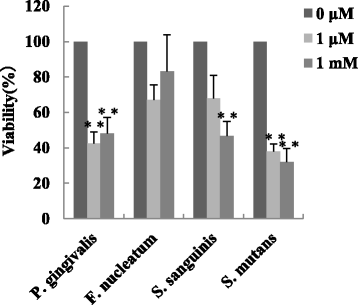
Results: Irradiation with the 310 nm UVB-LED at 105 mJ/cm2 showed 30-50% bactericidal activity to oral bacteria, though 17.1 mJ/cm2 irradiation with the 265 nm UVC-LED completely killed the bacteria. Ca9-22 cells were strongly injured by irradiation with the 265 nm UVC-LED but were not harmed by irradiation with the 310 nm UVB-LED. Nitric oxide and hydrogen peroxide were produced by Ca9-22 cells with irradiation using the 310 nm UVB-LED. P. gingivalis was killed by applying small amounts of those reactive oxygen species (ROS) in culture, but other bacteria showed low sensitivity to the ROS.
Conclusions: Narrowband UVB-LED irradiation exhibited a weak bactericidal effect on oral bacteria but showed low toxicity to gingival epithelial cells. Its irradiation also induces the production of ROS from oral epithelial cells and may enhance bactericidal activity to specific periodontopathic bacteria. It may be useful as a new adjunctive therapy for periodontitis.
Keywords: Periodontitis; Phototherapy; Porphyromonas gingivalis; Reactive oxygen species; Selective toxicity.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

