In vitro antineoplastic effects of brivaracetam and lacosamide on human glioma cells
- PMID: 28587680
- PMCID: PMC5460451
- DOI: 10.1186/s13046-017-0546-9
In vitro antineoplastic effects of brivaracetam and lacosamide on human glioma cells
Abstract
Background: Epilepsy is a frequent symptom in patients with glioma. Although treatment with antiepileptic drugs is generally effective in controlling seizures, drug-resistant patients are not uncommon. Multidrug resistance proteins (MRPs) and P-gp are over-represented in brain tissue of patients with drug-resistant epilepsy, suggesting their involvement in the clearance of antiepileptic medications. In addition to their anticonvulsant action, some drugs have been documented for cytotoxic effects. Aim of this study was to evaluate possible in vitro cytotoxic effects of two new-generation antiepileptic drugs on a human glioma cell line U87MG.
Methods: Cytotoxicity of brivaracetam and lacosamide was tested on U87MG, SW1783 and T98G by MTS assay. Expression of chemoresistance molecules was evaluated using flow cytometry in U87MG and human umbilical vein endothelial cells (HUVECs). To investigate the putative anti-proliferative effect, apoptosis assay, microRNA expression profile and study of cell cycle were performed.
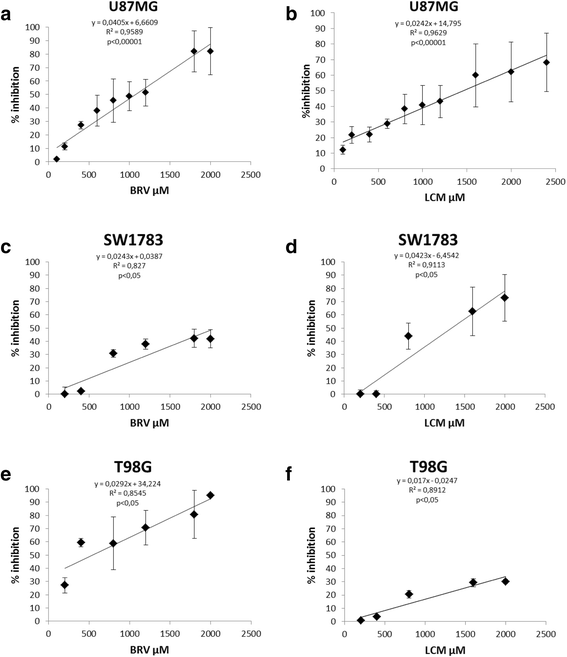
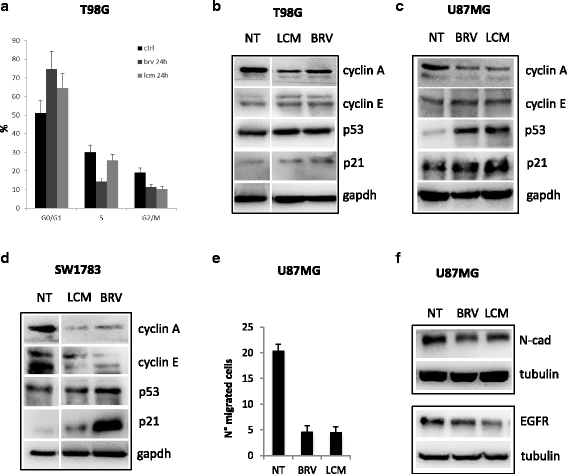
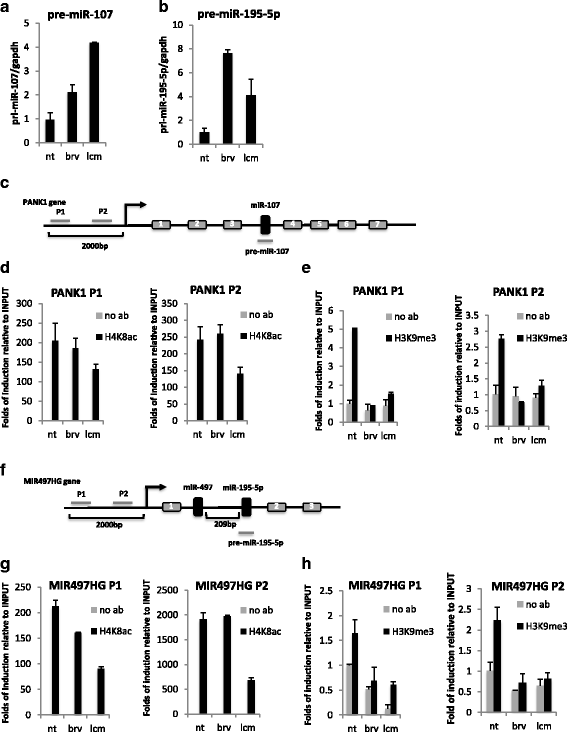
Results: Brivaracetam and lacosamide showed a dose-dependent cytotoxic and anti-migratory effects. Cytotoxicity was not related to apoptosis. The exposure of glioma cells to brivaracetam and lacosamide resulted in the modulation of several microRNAs; particularly, the effect of miR-195-5p modulation seemed to affect cell cycle, while miR-107 seemed to be implicated in the inhibition of cells migration. Moreover, brivaracetam and lacosamide treatment did not modulate the expression of chemoresistance-related molecules MRPs1-3-5, GSTπ, P-gp on U87MG and HUVECs.
Conclusion: Based on antineoplastic effect of brivaracetam and lacosamide on glioma cells, we assume that patients with glioma could benefit by the treatment with these two molecules, in addition to standard therapeutic options.
Keywords: Brain tumor-related epilepsy; Brivaracetam; Cytotoxicity; Glioma; Lacosamide.
Figures



References
-
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, Dodel R, Ekman M, Faravelli C, Fratiglioni L, Gannon B, Jones DH, Jennum P, Jordanova A, Jonsson L, Karampampa K, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(10):718–79. doi: 10.1016/j.euroneuro.2011.08.008. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

