PLGF, a placental marker of fetal brain defects after in utero alcohol exposure
- PMID: 28587682
- PMCID: PMC5461764
- DOI: 10.1186/s40478-017-0444-6
PLGF, a placental marker of fetal brain defects after in utero alcohol exposure
Abstract
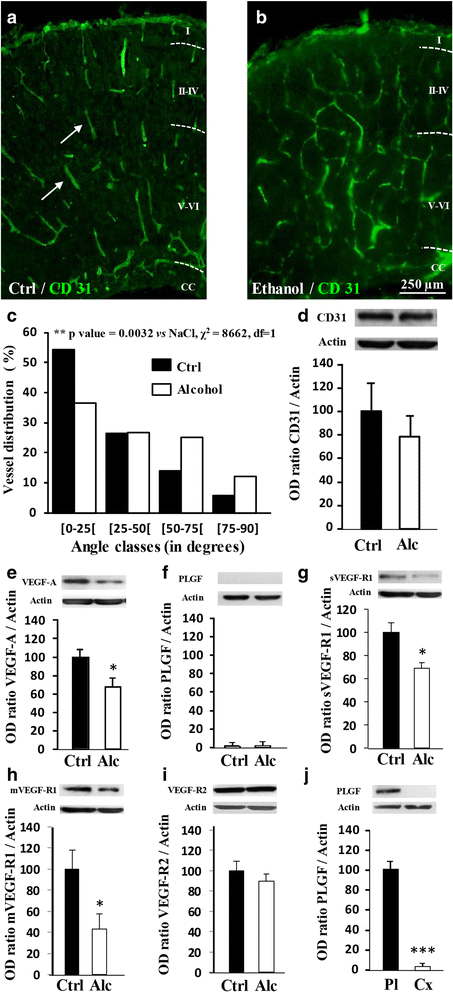
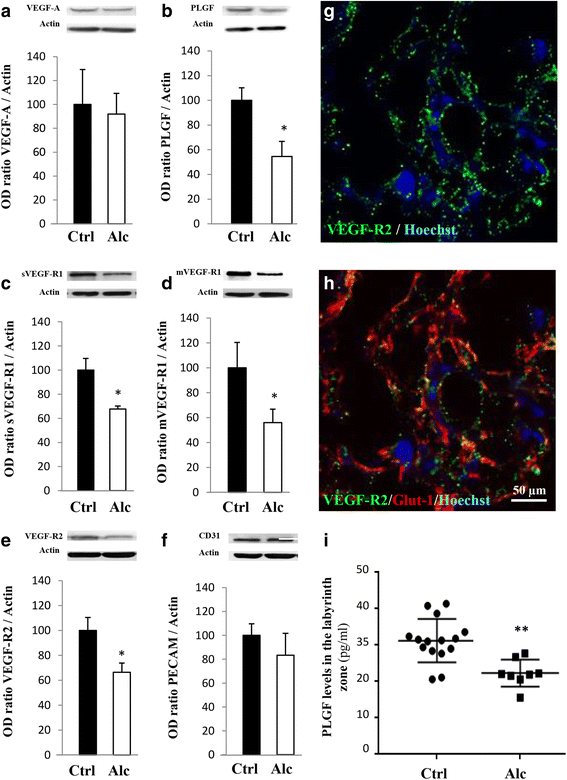
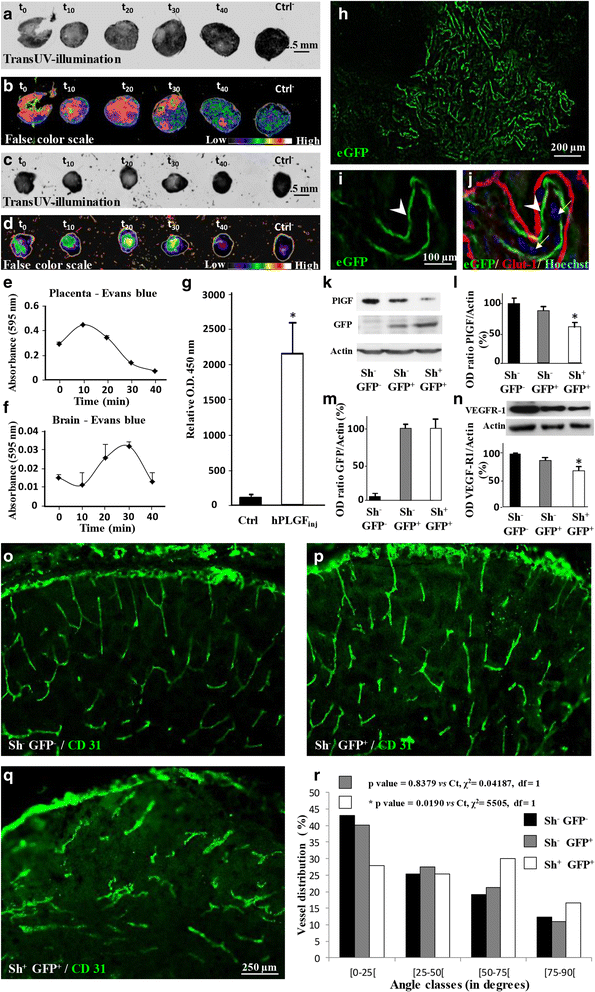
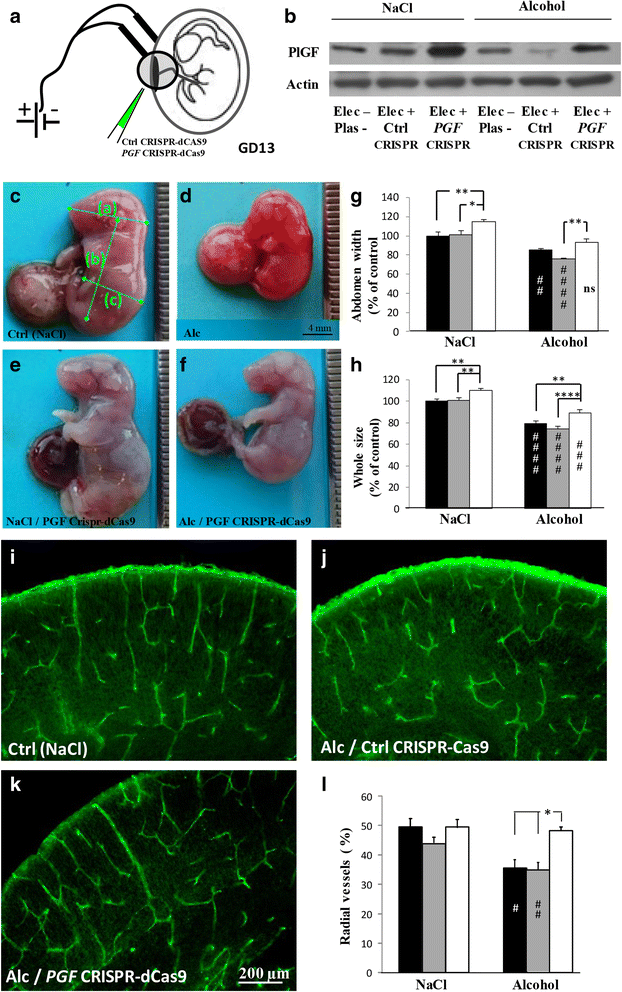
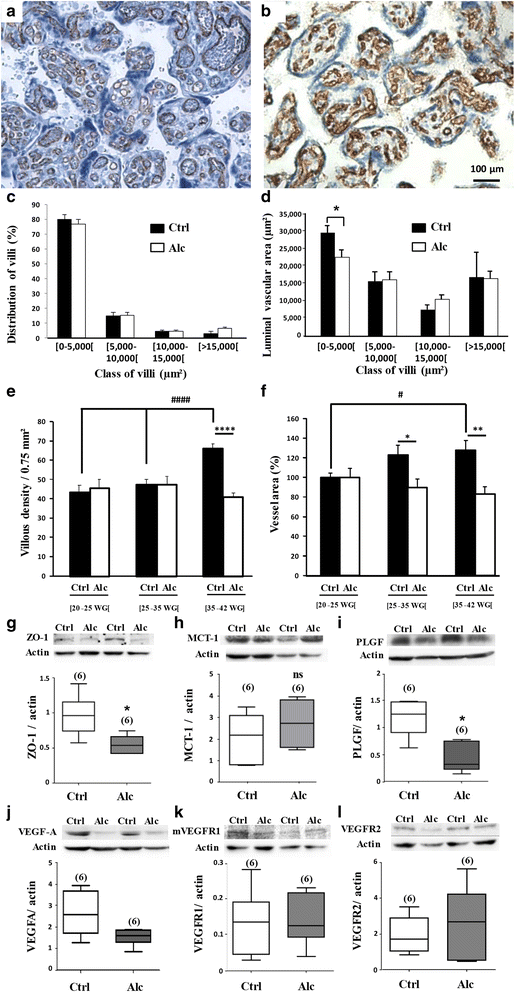
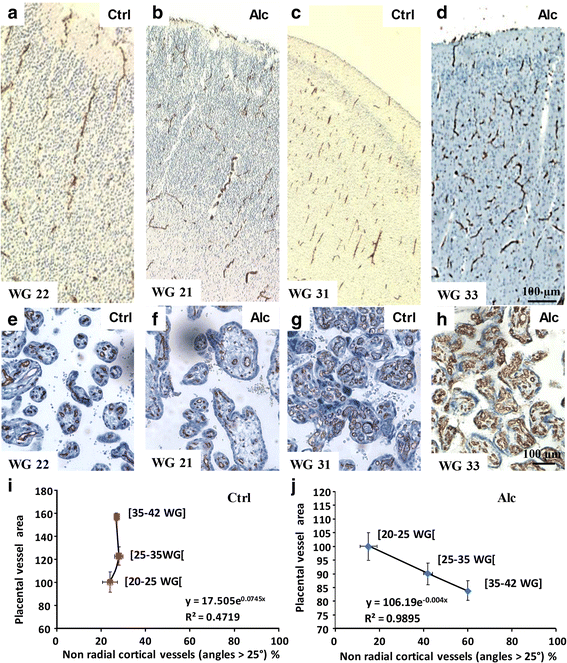
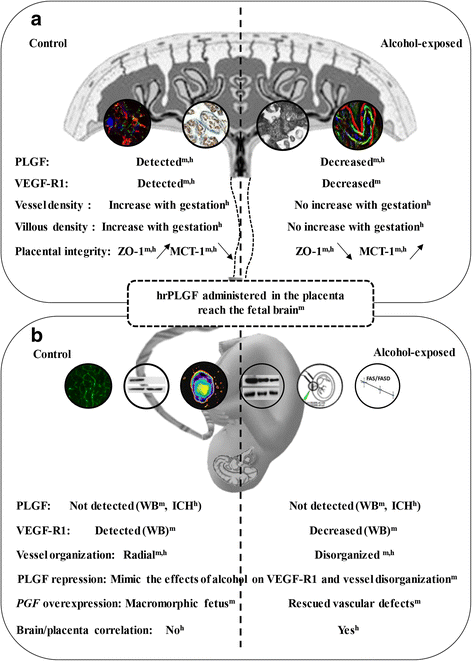
Most children with in utero alcohol exposure do not exhibit all features of fetal alcohol syndrome (FAS), and a challenge for clinicians is to make an early diagnosis of fetal alcohol spectrum disorders (FASD) to avoid lost opportunities for care. In brain, correct neurodevelopment requires proper angiogenesis. Since alcohol alters brain angiogenesis and the placenta is a major source of angiogenic factors, we hypothesized that it is involved in alcohol-induced brain vascular defects. In mouse, using in vivo repression and overexpression of PLGF, we investigated the contribution of placenta on fetal brain angiogenesis. In human, we performed a comparative molecular and morphological analysis of brain/placenta angiogenesis in alcohol-exposed fetuses. Results showed that prenatal alcohol exposure impairs placental angiogenesis, reduces PLGF levels and consequently alters fetal brain vasculature. Placental repression of PLGF altered brain VEGF-R1 expression and mimicked alcohol-induced vascular defects in the cortex. Over-expression of placental PGF rescued alcohol effects on fetal brain vessels. In human, alcohol exposure disrupted both placental and brain angiogenesis. PLGF expression was strongly decreased and angiogenesis defects observed in the fetal brain markedly correlated with placental vascular impairments. Placental PGF disruption impairs brain angiogenesis and likely predicts brain disabilities after in utero alcohol exposure. PLGF assay at birth could contribute to the early diagnosis of FASD.
Keywords: Angiogenesis; Cortex; Fetal alcohol exposure; Placenta.
Figures
Similar articles
-
Placental growth factor deficiency is associated with impaired cerebral vascular development in mice.Mol Hum Reprod. 2016 Feb;22(2):130-42. doi: 10.1093/molehr/gav069. Epub 2015 Dec 7. Mol Hum Reprod. 2016. PMID: 26646502 Free PMC article.
-
Epigallocatechin Gallate Ameliorates the Effects of Prenatal Alcohol Exposure in a Fetal Alcohol Spectrum Disorder-Like Mouse Model.Int J Mol Sci. 2021 Jan 13;22(2):715. doi: 10.3390/ijms22020715. Int J Mol Sci. 2021. PMID: 33450816 Free PMC article.
-
Prenatal Alcohol Exposure Impairs the Placenta-Cortex Transcriptomic Signature, Leading to Dysregulation of Angiogenic Pathways.Int J Mol Sci. 2023 Aug 30;24(17):13484. doi: 10.3390/ijms241713484. Int J Mol Sci. 2023. PMID: 37686296 Free PMC article.
-
[Fetal alcohol exposure: when placenta would help to the early diagnosis of child brain impairments].Med Sci (Paris). 2019 Nov;35(11):859-865. doi: 10.1051/medsci/2019167. Epub 2019 Dec 17. Med Sci (Paris). 2019. PMID: 31845877 Review. French.
-
Potential roles of imprinted genes in the teratogenic effects of alcohol on the placenta, somatic growth, and the developing brain.Exp Neurol. 2022 Jan;347:113919. doi: 10.1016/j.expneurol.2021.113919. Epub 2021 Nov 6. Exp Neurol. 2022. PMID: 34752786 Review.
Cited by
-
Mouse In Vivo Placental Targeted CRISPR Manipulation.J Vis Exp. 2023 Apr 14;(194):10.3791/64760. doi: 10.3791/64760. J Vis Exp. 2023. PMID: 37125793 Free PMC article.
-
Placental Igf1 Overexpression Sex-Specifically Impacts Mouse Placenta Structure, Altering Offspring Striatal Development and Behavior.bioRxiv [Preprint]. 2025 Mar 28:2025.03.27.644829. doi: 10.1101/2025.03.27.644829. bioRxiv. 2025. PMID: 40196637 Free PMC article. Preprint.
-
Nonconceptus Mechanisms of Prenatal Alcohol Exposure That Disrupt Embryo-Fetal Development: An Integrative View.Alcohol Res. 2025 Jul 16;45(1):07. doi: 10.35946/arcr.v45.1.07. eCollection 2025. Alcohol Res. 2025. PMID: 40692661 Free PMC article. Review.
-
Future Horizons for Neurodevelopmental Disorders: Placental Mechanisms.Front Pediatr. 2021 Apr 8;9:653230. doi: 10.3389/fped.2021.653230. eCollection 2021. Front Pediatr. 2021. PMID: 33898362 Free PMC article. No abstract available.
-
Effects of placental growth factor deficiency on behavior, neuroanatomy, and cerebrovasculature of mice.Physiol Genomics. 2018 Oct 1;50(10):862-875. doi: 10.1152/physiolgenomics.00076.2018. Epub 2018 Aug 17. Physiol Genomics. 2018. PMID: 30118404 Free PMC article.
References
-
- Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

