Functional communication between PKC-targeted cardiac troponin I phosphorylation sites
- PMID: 28587770
- PMCID: PMC5555030
- DOI: 10.1016/j.abb.2017.05.019
Functional communication between PKC-targeted cardiac troponin I phosphorylation sites
Abstract
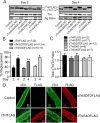
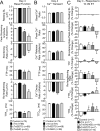
Increased protein kinase C (PKC) activity is associated with heart failure, and can target multiple cardiac troponin I (cTnI) residues in myocytes, including S23/24, S43/45 and T144. In earlier studies, cTnI-S43D and/or -S45D augmented S23/24 and T144 phosphorylation, which suggested there is communication between clusters. This communication is now explored by evaluating the impact of phospho-mimetic cTnI S43/45D combined with S23/24D (cTnIS4D) or T144D (cTnISDTD). Gene transfer of epitope-tagged cTnIS4D and cTnISDTD into adult cardiac myocytes progressively replaced endogenous cTnI. Partial replacement with cTnISDTD or cTnIS4D accelerated the time to peak (TTP) shortening and time to 50% re-lengthening (TTR50%) on day 2, but peak shortening was only diminished by cTnIS4D. Extensive cTnIS4D replacement continued to accelerate TTP, and decrease shortening amplitude, while TTR50% returned to baseline levels on day 4. In contrast, cTnISDTD modestly reduced shortening amplitude and continued to accelerate myocyte TTP and TTR50%. These results indicate cTnIS43/45 communicates with S23/24 and T144, with S23/24 exacerbating and T144 attenuating the S43/45D-dependent functional deficit. In addition, more severe functional alterations in cTnIS4D myocytes were accompanied by higher levels of secondary phosphorylation compared to cTnISDTD. These results suggest that secondary phosphorylation helps to maintain steady-state contractile function during chronic cTnI phosphorylation at PKC sites.
Keywords: Cardiac myocyte; Contractile function; Myofilament; Phosphorylation; Troponin I.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




References
-
- Andrew CD, Warwicker J, Jones GR, Doig AJ. Effect of phosphorylation on alpha-helix stability as a function of position. Biochemistry. 2002;41:1897–1905. - PubMed
-
- Bayer AL, Heidkamp MC, Patel N, Porter M, Engman S, Samarel AM. Alterations in protein kinase C isoenzyme expression and autophosphorylation during the progression of pressure overload-induced left ventricular hypertrophy. Mol Cell Biochem. 2003;242:145–152. - PubMed
-
- Bilchick KC, Duncan JG, Ravi R, Takimoto E, Champion HC, Gao WD, Stull LB, Kass DA, Murphy AM. Heart failure-associated alterations in troponin I phosphorylation impair ventricular relaxation-afterload and force-frequency responses and systolic function. Am J Physiol Heart Circ Physiol. 2007;292:H318–325. - PubMed
-
- Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, Mintze K, Pickard T, Roden R, Bristow MR, Sabbah HN, Mizrahi JL, Gromo G, King GL, Vlahos CJ. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–391. - PubMed
-
- Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

