A Medical Student-Delivered Smoking Prevention Program, Education Against Tobacco, for Secondary Schools in Germany: Randomized Controlled Trial
- PMID: 28588007
- PMCID: PMC5478798
- DOI: 10.2196/jmir.7906
A Medical Student-Delivered Smoking Prevention Program, Education Against Tobacco, for Secondary Schools in Germany: Randomized Controlled Trial
Abstract
Background: More than 8.5 million Germans suffer from chronic diseases attributable to smoking. Education Against Tobacco (EAT) is a multinational network of medical students who volunteer for school-based prevention in the classroom setting, amongst other activities. EAT has been implemented in 28 medical schools in Germany and is present in 13 additional countries around the globe. A recent quasi-experimental study showed significant short-term smoking cessation effects on 11-to-15-year-old adolescents.
Objective: The aim of this study was to provide the first randomized long-term evaluation of the optimized 2014 EAT curriculum involving a photoaging software for its effectiveness in reducing the smoking prevalence among 11-to-15-year-old pupils in German secondary schools.
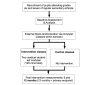
Methods: A randomized controlled trial was undertaken with 1504 adolescents from 9 German secondary schools, aged 11-15 years in grades 6-8, of which 718 (47.74%) were identifiable for the prospective sample at the 12-month follow-up. The experimental study design included measurements at baseline (t1), 6 months (t2), and 12 months postintervention (t3), via questionnaire. The study groups consisted of 40 randomized classes that received the standardized EAT intervention (two medical student-led interactive modules taking 120 minutes total) and 34 control classes within the same schools (no intervention). The primary endpoint was the difference in smoking prevalence from t1 to t3 in the control group versus the difference from t1 to t3 in the intervention group. The differences in smoking behavior (smoking onset, quitting) between the two groups, as well as gender-specific effects, were studied as secondary outcomes.
Results: None of the effects were significant due to a high loss-to-follow-up effect (52.26%, 786/1504). From baseline to the two follow-up time points, the prevalence of smoking increased from 3.1% to 5.2% to 7.2% in the control group and from 3.0% to 5.4% to 5.8% in the intervention group (number needed to treat [NNT]=68). Notable differences were observed between the groups for the female gender (4.2% to 9.5% for control vs 4.0% to 5.2% for intervention; NNT=24 for females vs NNT=207 for males), low educational background (7.3% to 12% for control vs 6.1% to 8.7% for intervention; NNT=30), and migrational background (students who claimed that at least one parent was not born in Germany) at the 12-month follow-up. The intervention appears to prevent smoking onset (NNT=63) but does not appear to initiate quitting.
Conclusions: The intervention appears to prevent smoking, especially in females and students with a low educational background.
Keywords: adolescents; medical students; school-based prevention; secondary schools; smoking cessation; tobacco prevention.
©Titus Josef Brinker, Andreas Dawid Owczarek, Werner Seeger, David Alexander Groneberg, Christian Martin Brieske, Philipp Jansen, Joachim Klode, Ingo Stoffels, Dirk Schadendorf, Benjamin Izar, Fabian Norbert Fries, Felix Johannes Hofmann. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 06.06.2017.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- Petrescu D, Vasiljevic M, Pepper J, Ribisl K, Marteau TM. What is the impact of e-cigarette adverts on children's perceptions of tobacco smoking? An experimental study. Tob Control. 2016 Sep 05;:5–8. doi: 10.1136/tobaccocontrol-2016-052940. http://tobaccocontrol.bmj.com/cgi/pmidlookup?view=long&pmid=27601455 - DOI - PMC - PubMed
-
- Mons U, Müezzinler Aysel, Gellert C, Schöttker Ben, Abnet C, Bobak M, de Groot L, Freedman N, Jansen E, Kee F, Kromhout D, Kuulasmaa K, Laatikainen T, O'Doherty MG, Bueno-de-Mesquita B, Orfanos P, Peters A, van der Schouw YT, Wilsgaard T, Wolk A, Trichopoulou A, Boffetta P, Brenner H. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015 Apr 20;350:h1551. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=25896935 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

