Novel Chromosome Organization Pattern in Actinomycetales-Overlapping Replication Cycles Combined with Diploidy
- PMID: 28588128
- PMCID: PMC5461407
- DOI: 10.1128/mBio.00511-17
Novel Chromosome Organization Pattern in Actinomycetales-Overlapping Replication Cycles Combined with Diploidy
Abstract
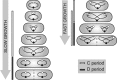
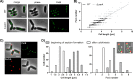
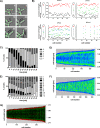
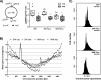
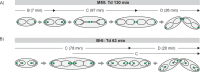
Bacteria regulate chromosome replication and segregation tightly with cell division to ensure faithful segregation of DNA to daughter generations. The underlying mechanisms have been addressed in several model species. It became apparent that bacteria have evolved quite different strategies to regulate DNA segregation and chromosomal organization. We have investigated here how the actinobacterium Corynebacterium glutamicum organizes chromosome segregation and DNA replication. Unexpectedly, we found that C. glutamicum cells are at least diploid under all of the conditions tested and that these organisms have overlapping C periods during replication, with both origins initiating replication simultaneously. On the basis of experimental data, we propose growth rate-dependent cell cycle models for C. glutamicumIMPORTANCE Bacterial cell cycles are known for few model organisms and can vary significantly between species. Here, we studied the cell cycle of Corynebacterium glutamicum, an emerging cell biological model organism for mycolic acid-containing bacteria, including mycobacteria. Our data suggest that C. glutamicum carries two pole-attached chromosomes that replicate with overlapping C periods, thus initiating a new round of DNA replication before the previous one is terminated. The newly replicated origins segregate to midcell positions, where cell division occurs between the two new origins. Even after long starvation or under extremely slow-growth conditions, C. glutamicum cells are at least diploid, likely as an adaptation to environmental stress that may cause DNA damage. The cell cycle of C. glutamicum combines features of slow-growing organisms, such as polar origin localization, and fast-growing organisms, such as overlapping C periods.
Keywords: Corynebacterium; ParA; ParB; cell cycle; diploidy; origin; replication.
Copyright © 2017 Böhm et al.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
