The Rcs-Regulated Colanic Acid Capsule Maintains Membrane Potential in Salmonella enterica serovar Typhimurium
- PMID: 28588134
- PMCID: PMC5461412
- DOI: 10.1128/mBio.00808-17
The Rcs-Regulated Colanic Acid Capsule Maintains Membrane Potential in Salmonella enterica serovar Typhimurium
Abstract
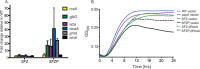
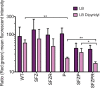
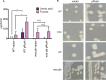
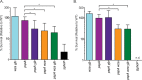
The Rcs phosphorelay and Psp (phage shock protein) systems are envelope stress responses that are highly conserved in gammaproteobacteria. The Rcs regulon was found to be strongly induced during metal deprivation of Salmonella enterica serovar Typhimurium lacking the Psp response. Nineteen genes activated by the RcsA-RcsB response regulator make up an operon responsible for the production of colanic acid capsular polysaccharide, which promotes biofilm development. Despite more than half a century of research, the physiological function of colanic acid has remained elusive. Here we show that Rcs-dependent colanic acid production maintains the transmembrane electrical potential and proton motive force in cooperation with the Psp response. Production of negatively charged exopolysaccharide covalently bound to the outer membrane may enhance the surface potential by increasing the local proton concentration. This provides a unifying mechanism to account for diverse Rcs/colanic acid-related phenotypes, including susceptibility to membrane-damaging agents and biofilm formation.IMPORTANCE Colanic acid is a negatively charged polysaccharide capsule produced by Escherichia coli, Salmonella, and other gammaproteobacteria. Research conducted over the 50 years since the discovery of colanic acid suggests that this exopolysaccharide plays an important role for bacteria living in biofilms. However, a precise physiological role for colanic acid has not been defined. In this study, we provide evidence that colanic acid maintains the transmembrane potential and proton motive force during envelope stress. This work provides a new and fundamental insight into bacterial physiology.
Keywords: Salmonella; biofilms; colanic acid; exopolysaccharide; extracytoplasmic stress; proton motive force.
Copyright © 2017 Pando et al.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

