Gut Dysbiosis and Neurobehavioral Alterations in Rats Exposed to Silver Nanoparticles
- PMID: 28588204
- PMCID: PMC5460200
- DOI: 10.1038/s41598-017-02880-0
Gut Dysbiosis and Neurobehavioral Alterations in Rats Exposed to Silver Nanoparticles
Abstract
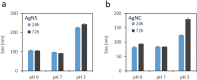
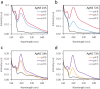
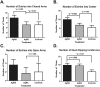
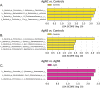
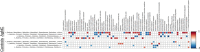
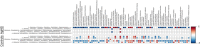
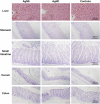
Due to their antimicrobial properties, silver nanoparticles (AgNPs) are being used in non-edible and edible consumer products. It is not clear though if exposure to these chemicals can exert toxic effects on the host and gut microbiome. Conflicting studies have been reported on whether AgNPs result in gut dysbiosis and other changes within the host. We sought to examine whether exposure of Sprague-Dawley male rats for two weeks to different shapes of AgNPs, cube (AgNC) and sphere (AgNS) affects gut microbiota, select behaviors, and induces histopathological changes in the gastrointestinal system and brain. In the elevated plus maze (EPM), AgNS-exposed rats showed greater number of entries into closed arms and center compared to controls and those exposed to AgNC. AgNS and AgNC treated groups had select reductions in gut microbiota relative to controls. Clostridium spp., Bacteroides uniformis, Christensenellaceae, and Coprococcus eutactus were decreased in AgNC exposed group, whereas, Oscillospira spp., Dehalobacterium spp., Peptococcaeceae, Corynebacterium spp., Aggregatibacter pneumotropica were reduced in AgNS exposed group. Bacterial reductions correlated with select behavioral changes measured in the EPM. No significant histopathological changes were evident in the gastrointestinal system or brain. Findings suggest short-term exposure to AgNS or AgNC can lead to behavioral and gut microbiome changes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

