High-resolution promoter map of human limbal epithelial cells cultured with keratinocyte growth factor and rho kinase inhibitor
- PMID: 28588247
- PMCID: PMC5460231
- DOI: 10.1038/s41598-017-02824-8
High-resolution promoter map of human limbal epithelial cells cultured with keratinocyte growth factor and rho kinase inhibitor
Abstract
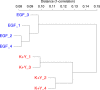
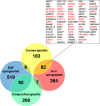
An in vitro model of corneal epithelial cells (CECs) has been developed to study and treat corneal disorders. Nevertheless, conventional CEC culture supplemented with epidermal growth factor (EGF) results in a loss of CEC characteristics. It has recently been reported that limbal epithelial cells (LECs) cultured with keratinocyte growth factor (KGF) and the rho kinase inhibitor Y-27632 could maintain the expression of several CEC-specific markers. However, the molecular mechanism underlying the effect of culture media on LECs remains to be elucidated. To elucidate this mechanism, we performed comprehensive gene expression analysis of human LECs cultured with EGF or KGF/Y-27632, by cap analysis of gene expression (CAGE). Here, we found that LECs cultured with KGF and Y-27632 presented a gene expression profile highly similar to that of CECs in vivo. In contrast, LECs cultured with EGF lost the characteristic CEC gene expression profile. We further discovered that CEC-specific PAX6 promoters are highly activated in LECs cultured with KGF and Y-27632. Our results provide strong evidence that LECs cultured with KGF and Y-27632 would be an improved in vitro model in the context of gene expression. These findings will accelerate basic studies of CECs and clinical applications in regenerative medicine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor.Stem Cells Transl Med. 2013 Oct;2(10):758-65. doi: 10.5966/sctm.2012-0156. Epub 2013 Aug 27. Stem Cells Transl Med. 2013. PMID: 23981725 Free PMC article.
-
Differential regulation of keratinocyte growth factor and hepatocyte growth factor/scatter factor by different cytokines in human corneal and limbal fibroblasts.J Cell Physiol. 1997 Sep;172(3):361-72. doi: 10.1002/(SICI)1097-4652(199709)172:3<361::AID-JCP10>3.0.CO;2-9. J Cell Physiol. 1997. PMID: 9284956
-
The growth-promoting effect of KGF on limbal epithelial cells is mediated by upregulation of DeltaNp63alpha through the p38 pathway.J Cell Sci. 2009 Dec 15;122(Pt 24):4473-80. doi: 10.1242/jcs.054791. Epub 2009 Nov 17. J Cell Sci. 2009. PMID: 19920075
-
The effect of culture medium and carrier on explant culture of human limbal epithelium: A comparison of ultrastructure, keratin profile and gene expression.Exp Eye Res. 2016 Dec;153:122-132. doi: 10.1016/j.exer.2016.09.012. Epub 2016 Oct 1. Exp Eye Res. 2016. PMID: 27702552
-
Identification and characterization of limbal stem cells.Exp Eye Res. 2005 Sep;81(3):247-64. doi: 10.1016/j.exer.2005.02.016. Exp Eye Res. 2005. PMID: 16051216 Review.
Cited by
-
PAX6-WNK2 Axis Governs Corneal Epithelial Homeostasis.Invest Ophthalmol Vis Sci. 2024 Oct 1;65(12):40. doi: 10.1167/iovs.65.12.40. Invest Ophthalmol Vis Sci. 2024. PMID: 39453672 Free PMC article.
-
Corneal Epithelial Stem Cells-Physiology, Pathophysiology and Therapeutic Options.Cells. 2021 Sep 3;10(9):2302. doi: 10.3390/cells10092302. Cells. 2021. PMID: 34571952 Free PMC article. Review.
-
The secretome of adipose-derived mesenchymal stem cells attenuates epithelial-mesenchymal transition in human corneal epithelium.Regen Ther. 2019 Jul 2;11:114-122. doi: 10.1016/j.reth.2019.06.005. eCollection 2019 Dec. Regen Ther. 2019. PMID: 31312693 Free PMC article.
-
KLF4 prevents epithelial to mesenchymal transition in human corneal epithelial cells via endogenous TGF-β2 suppression.Regen Ther. 2019 Sep 12;11:249-257. doi: 10.1016/j.reth.2019.08.003. eCollection 2019 Dec. Regen Ther. 2019. PMID: 31538102 Free PMC article.
-
PAX6-dependent differentiation landscape of ocular surface epithelium via single-cell RNA sequencing in hiPSC-derived ocular developmental organoid.Commun Biol. 2025 Aug 14;8(1):1220. doi: 10.1038/s42003-025-08643-2. Commun Biol. 2025. PMID: 40813888 Free PMC article.
References
-
- Cenedella RJ, Fleschner CR. Kinetics of corneal epithelium turnover Studies of lovastatinin vivo. Invest Ophthalmol Vis Sci. 1990;31:1957–1962. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

