Pre- and post-diagnostic β-blocker use and lung cancer survival: A population-based cohort study
- PMID: 28588274
- PMCID: PMC5460218
- DOI: 10.1038/s41598-017-02913-8
Pre- and post-diagnostic β-blocker use and lung cancer survival: A population-based cohort study
Abstract
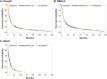
Beta-blockers have been associated with decreased cancer mortality. However, evidence for lung cancer is sparse and reported beneficial effects might be based on biased analyses. In this so far largest study we investigated the association between β-blocker use and lung cancer survival. Therefore, patients with a lung cancer diagnosis between April 1998 and December 2011 were selected from a database linkage of the Netherlands Cancer Registry and the PHARMO Database Network. After matching eligible patients on the propensity score, adjusted hazard ratios (HRs) and corresponding 95% confidence intervals (CI) were calculated using Cox proportional hazards regression to investigate the association between pre-diagnostic and time-dependent β-blocker use and overall survival. Duration and dose-response analyses and stratified analyses by β-blocker type, histological subgroups and stage were conducted. Of 3,340 eligible lung cancer patients, 1437 (43%) took β-blockers four months prior to diagnosis. Pre-diagnostic β-blocker use was not associated with overall survival (HR 1.00 (0.92-1.08)) in the adjusted model. Time-dependent post-diagnostic analysis showed similar results with a HR of 1.03 (0.94-1.11). Trend analyses showed no association for cumulative dose (HR 0.99 (0.97-1.02)) and cumulative duration (HR 1.00 (0.96-1.05)). In conclusion, β-blocker use is not associated with reduced mortality among lung cancer patients.
Conflict of interest statement
J.W., L.J., M.H., P.V. and M.W. have nothing to disclaim. H.B. reports grants from the German Cancer Aid and from German Federal Ministry of Education and Research, during the conduct of the study. M.H.S. is an employee of the PHARMO Institute for Drug Outcomes Research; this independent research institute performs financially supported studies for government and related healthcare authorities and several pharmaceutical companies. W.H. reports personal fees and non-financial support from Aqua Institute Göttingen, Aspen Europe GmbH, Diaplan, EvalueScience Ltd., Grünenthal GmbH, GSK GER/UK/Slovakia/France/Espana/Poland, and Novartis, other from Dosing GmbH, personal fees from Actelion GmbH, AstraZenica GmbH, Berlin-Chemie AG, Boehringer GmbH, Bristol-Myers Squibb GmbH, Gesundheitsamt Österreich, KWHC GmbH, MSD Sharp & Dohme GmbH, Roche, UK, and Südwestrundfunk, personal fees and other from Thieme Verlag and Daiichi Sankyo GmbH, grants and personal fees from Landesapothekerkammer Hessen/Nieders/BW, grants from BMBF (DZIF, ESTHER), EU (QUALMAT), outside the submitted work; and WEH is a member of the scientific advisory board and shareholder of Dosing GmbH, the company distributing the clinical decision support software used in this study. His wife is an employee of Dosing GmbH.
Figures
Similar articles
-
Immortal time bias in pharmacoepidemiological studies on cancer patient survival: empirical illustration for beta-blocker use in four cancers with different prognosis.Eur J Epidemiol. 2017 Nov;32(11):1019-1031. doi: 10.1007/s10654-017-0304-5. Epub 2017 Sep 1. Eur J Epidemiol. 2017. PMID: 28864947
-
Pre- and post-diagnostic beta-blocker use and prognosis after colorectal cancer: Results from a population-based study.Int J Cancer. 2017 Jul 1;141(1):62-71. doi: 10.1002/ijc.30717. Int J Cancer. 2017. PMID: 28370155
-
β-Blocker use and all-cause mortality of melanoma patients: results from a population-based Dutch cohort study.Eur J Cancer. 2013 Dec;49(18):3863-71. doi: 10.1016/j.ejca.2013.07.141. Epub 2013 Aug 10. Eur J Cancer. 2013. PMID: 23942335
-
Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies.Br J Anaesth. 2018 Jul;121(1):45-57. doi: 10.1016/j.bja.2018.03.024. Epub 2018 May 3. Br J Anaesth. 2018. PMID: 29935594
-
Treatment disparities for disabled medicare beneficiaries with stage I non-small cell lung cancer.Arch Phys Med Rehabil. 2008 Apr;89(4):595-601. doi: 10.1016/j.apmr.2007.09.042. Arch Phys Med Rehabil. 2008. PMID: 18373987 Review.
Cited by
-
Repurposing established cyclic adenosine monophosphate reducing agents for the prevention and therapy of epidermal growth factor receptor inhibitor resistance in non-small cell lung cancer.Transl Lung Cancer Res. 2018 Apr;7(Suppl 2):S117-S122. doi: 10.21037/tlcr.2018.03.04. Transl Lung Cancer Res. 2018. PMID: 29782563 Free PMC article. No abstract available.
-
Chronic Stress Effects on Tumor: Pathway and Mechanism.Front Oncol. 2021 Dec 20;11:738252. doi: 10.3389/fonc.2021.738252. eCollection 2021. Front Oncol. 2021. PMID: 34988010 Free PMC article. Review.
-
Antidiabetics, Anthelmintics, Statins, and Beta-Blockers as Co-Adjuvant Drugs in Cancer Therapy.Medicina (Kaunas). 2022 Sep 7;58(9):1239. doi: 10.3390/medicina58091239. Medicina (Kaunas). 2022. PMID: 36143915 Free PMC article. Review.
-
Cardiovascular Disease and Cancer: Is There Increasing Overlap?Curr Oncol Rep. 2019 Apr 6;21(6):47. doi: 10.1007/s11912-019-0796-0. Curr Oncol Rep. 2019. PMID: 30955114 Review.
-
Beta-blocker and survival in patients with lung cancer: A meta-analysis.PLoS One. 2021 Feb 16;16(2):e0245773. doi: 10.1371/journal.pone.0245773. eCollection 2021. PLoS One. 2021. PMID: 33592015 Free PMC article.
References
-
- Partecke, L. I. et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology, doi:10.1016/j.pan.2016.03.005 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous