A Central Small Amino Acid in the VAMP2 Transmembrane Domain Regulates the Fusion Pore in Exocytosis
- PMID: 28588281
- PMCID: PMC5460238
- DOI: 10.1038/s41598-017-03013-3
A Central Small Amino Acid in the VAMP2 Transmembrane Domain Regulates the Fusion Pore in Exocytosis
Abstract
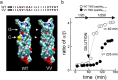
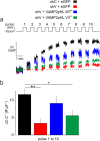
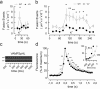
Exocytosis depends on cytosolic domains of SNARE proteins but the function of the transmembrane domains (TMDs) in membrane fusion remains controversial. The TMD of the SNARE protein synaptobrevin2/VAMP2 contains two highly conserved small amino acids, G100 and C103, in its central portion. Substituting G100 and/or C103 with the β-branched amino acid valine impairs the structural flexibility of the TMD in terms of α-helix/β-sheet transitions in model membranes (measured by infrared reflection-absorption or evanescent wave spectroscopy) during increase in protein/lipid ratios, a parameter expected to be altered by recruitment of SNAREs at fusion sites. This structural change is accompanied by reduced membrane fluidity (measured by infrared ellipsometry). The G100V/C103V mutation nearly abolishes depolarization-evoked exocytosis (measured by membrane capacitance) and hormone secretion (measured biochemically). Single-vesicle optical (by TIRF microscopy) and biophysical measurements of ATP release indicate that G100V/C103V retards initial fusion-pore opening, hinders its expansion and leads to premature closure in most instances. We conclude that the TMD of VAMP2 plays a critical role in membrane fusion and that the structural mobility provided by the central small amino acids is crucial for exocytosis by influencing the molecular re-arrangements of the lipid membrane that are necessary for fusion pore opening and expansion.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
The transmembrane domain of the SNARE protein VAMP2 is highly sensitive to its lipid environment.Biochim Biophys Acta Biomembr. 2019 Mar 1;1861(3):670-676. doi: 10.1016/j.bbamem.2018.12.011. Epub 2018 Dec 20. Biochim Biophys Acta Biomembr. 2019. PMID: 30579961
-
A structural role for the synaptobrevin 2 transmembrane domain in dense-core vesicle fusion pores.J Neurosci. 2015 Apr 8;35(14):5772-80. doi: 10.1523/JNEUROSCI.3983-14.2015. J Neurosci. 2015. PMID: 25855187 Free PMC article.
-
v-SNARE transmembrane domains function as catalysts for vesicle fusion.Elife. 2016 Jun 25;5:e17571. doi: 10.7554/eLife.17571. Elife. 2016. PMID: 27343350 Free PMC article.
-
Regulation of Exocytotic Fusion Pores by SNARE Protein Transmembrane Domains.Front Mol Neurosci. 2017 Oct 10;10:315. doi: 10.3389/fnmol.2017.00315. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29066949 Free PMC article. Review.
-
Fusion pore in exocytosis: More than an exit gate? A β-cell perspective.Cell Calcium. 2017 Dec;68:45-61. doi: 10.1016/j.ceca.2017.10.005. Epub 2017 Nov 7. Cell Calcium. 2017. PMID: 29129207 Review.
Cited by
-
Mechanisms of SNARE proteins in membrane fusion.Nat Rev Mol Cell Biol. 2024 Feb;25(2):101-118. doi: 10.1038/s41580-023-00668-x. Epub 2023 Oct 17. Nat Rev Mol Cell Biol. 2024. PMID: 37848589 Free PMC article. Review.
-
Forward genetics identifies a novel sleep mutant with sleep state inertia and REM sleep deficits.Sci Adv. 2020 Aug 12;6(33):eabb3567. doi: 10.1126/sciadv.abb3567. eCollection 2020 Aug. Sci Adv. 2020. PMID: 32851175 Free PMC article.
-
OutCyte: a novel tool for predicting unconventional protein secretion.Sci Rep. 2019 Dec 19;9(1):19448. doi: 10.1038/s41598-019-55351-z. Sci Rep. 2019. PMID: 31857603 Free PMC article.
-
Molecular mechanism underlying SNARE-mediated membrane fusion enlightened by all-atom molecular dynamics simulations.Proc Natl Acad Sci U S A. 2024 Apr 16;121(16):e2321447121. doi: 10.1073/pnas.2321447121. Epub 2024 Apr 9. Proc Natl Acad Sci U S A. 2024. PMID: 38593076 Free PMC article.
-
The exocyst complex and Rab5 are required for abscission by localizing ESCRT III subunits to the cytokinetic bridge.J Cell Sci. 2019 Jul 17;132(14):jcs226001. doi: 10.1242/jcs.226001. J Cell Sci. 2019. PMID: 31221728 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

